Summary: Closely related dopamine-releasing neurons in the olfactory bulb behave in fundamentally different ways depending on their physical structure. One subtype releases neurotransmitters from dendrites rather than axons, an unusual mechanism that allows cells to act locally and even self-inhibit.
Another subtype follows the classical neuronal model and uses long axons to send signals across distant regions. These structural differences mean that each cell type plays a different role in shaping how odors are processed, offering new insights into sensory coding and the diversity of neural communication.
Key facts:
Two distinct subtypes of dopaminergic neurons: one lacks an axon and releases neurotransmitters from dendrites; the other uses axons in the traditional way. Functional consequences: Anaxonic neurons act locally and self-inhibit, while axon-bearing neurons coordinate activity in regions distant from the olfactory bulb. Impact on sensory processing: These differences suggest that each subtype uniquely contributes to odor perception and contrast sharpening.
Source: King’s College London
Closely related subtypes of dopamine-releasing neurons may play completely separate roles in processing sensory information, depending on their physical structure.
New research from the Institute of Psychiatry, Psychology and Neuroscience (IoPPN) at King’s College London has found that variations in the physical structure of neurons could have a surprising impact on the role they play in processing sensory information.
He identified two different subtypes of interneurons in the olfactory bulb, which is where the brain first processes information about smell. One of these subtypes was found to communicate in a very unusual way, releasing signals from a part of the neuron that has most commonly been associated with receiving signals.
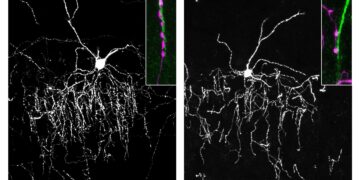
Published in eLife, the research examined the olfactory bulbs of mouse brains to assess the structure of different subtypes of neurons that produce and release the chemical dopamine.
It is the first study to provide anatomical and physiological evidence that different subtypes of dopaminergic neurons in the medulla transmit signals in fundamentally different ways depending on the shape and structure of the cell.
Most neurons send their signals by releasing chemical neurotransmitters through long, thin protrusions of the cell called axons. In the classical view, neurons also receive signals from other neurons through other types of branching, tree-like protrusions known as dendrites.
This distinction in the role played by these different structures forms the backbone of how neurons are thought to function. However, this new study provides evidence that these neuron structures may not always behave this way.
The researchers in this study discovered that neurons within the olfactory bulb can be separated into two distinct subtypes, clearly characterized by how they transmit and receive signals.
One type of dopamine interneuron in the olfactory bulb did not have any axons, but rather released neurotransmitter signals from its dendrites, usually the input rather than output part of the cell. These cells are called “anaxonic neurons.”
These unusual anaxonic neurons acted locally within the olfactory bulb and were able to self-inhibit, meaning they can reduce their own activity levels.
This study was the first to demonstrate that a separate subtype of neurons in the olfactory bulb that have axons, known as “axon-bearing dopaminergic neurons,” do not release signals from their dendrites and cannot self-inhibit.
These neurons followed the classical model of how neurons send signals to each other with the release sites contained almost entirely within the axon. These axons travel long distances through the olfactory bulb rather than influencing the electrical activity of their own cells through autoinhibition, like anaxonic neurons.
Dr Ana Dorrego-Rivas, postdoctoral researcher at King’s IoPPN and first author of the study, said: “Our findings support that the two dopaminergic subclasses play fundamentally different roles in the olfactory bulb.
“While axonless neurons act locally, shaping the processing of odor signals within specific spherical structures in the brain, axon-bearing cells act over long distances, coordinating activity between these spherical structures and potentially enhancing the contrast between different odors.
“Although both release dopamine and are located in the olfactory lobe, our findings suggest that these neurons contribute to olfactory processing in strikingly different ways.”
Professor Matthew Grubb, Professor of Neuroscience at King’s IoPPN and lead author of the study, said: “The olfactory system is weird and wonderful, so it was a big surprise to find some cells that behave like ‘standard’ neurons. It will be fun to try to discover how these abnormally normal cells contribute to the perception of olfactory stimuli.”
Funding: This research was funded by the Wellcome Trust, the European Research Council and UKRI through the Biotechnology and Biological Sciences Research Council and the Medical Research Council.
Key questions answered:
A: Their physical design determines where and how they release neurotransmitters, which determines their role in processing odor signals.
A: They release neurotransmitters from dendrites (normally input structures) and can attenuate their own activity through autoinhibition.
A: They transmit signals over longer distances, which helps coordinate activity across the olfactory bulb and refines odor discrimination.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this news about research on dopamine and smell
Author: Patrick O’Brien
Source: King’s College London
Contact: Patrick O’Brien – King’s College London
Image: Image is credited to Neuroscience News.
Original research: Open access.
“Surprisingly different neurotransmitter release strategies in dopaminergic subclasses” by Ana Dorrego-Rivas et al. eLife
Abstract
Strikingly different neurotransmitter release strategies in dopaminergic subclasses
Neuronal function is closely linked to axodendritic polarity. The release of neurotransmitters, for example, is usually the responsibility of the axon. However, there are widespread exceptions to this rule, including many mammalian neuronal types that can release neurotransmitters from their dendrites.
In the mouse olfactory bulb, closely related subclasses of dopaminergic interneurons differ markedly in their polarity, with one subtype lacking an axon entirely. These axon-bearing and anaxonic dopaminergic subclasses have distinct developmental profiles and sensory responses, but how their fundamental polarity differences translate into functional outcomes remains completely unknown.
Here, we provide anatomical evidence for distinct neurotransmitter release strategies among these closely related dopaminergic subtypes: anaxonal cells are released from their dendrites, while axon-bearing neurons are released exclusively from their intermittently myelinated axon.
These structural differences are linked to a clear functional distinction: anaxonic dopaminergic neurons, but not those containing axons, are capable of self-inhibition.
Our findings suggest that variations in polarity can produce striking distinctions in neuronal output, and that even closely related neuronal subclasses can play completely separate roles in the processing of sensory information.