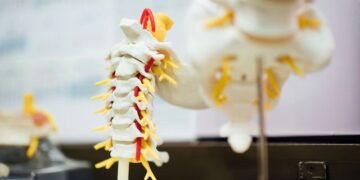
After almost three years of review, the Agência Nacional de Vigilância Sanitária (Anvisa) of Brazil announced the approval of the phase 1 clinical trial of polylaminin, a drug developed by researchers at the Federal University of Rio de Janeiro (UFRJ). According to the research team, the substance has shown the ability to regenerate spinal cord injuries. The new phase aims to evaluate the safety of its use in people with complete spinal cord injuries.
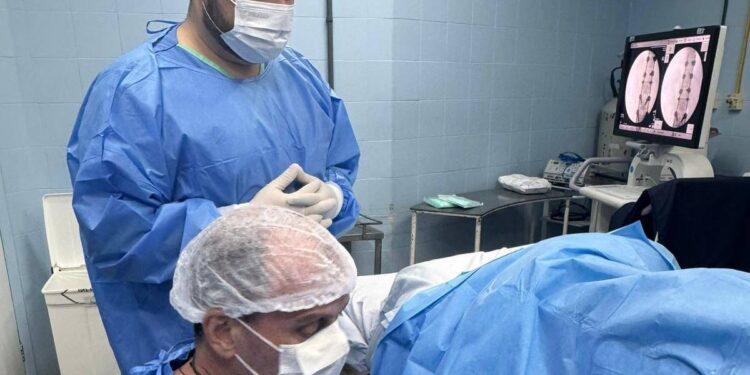
The pharmaceutical company Cristália will select five volunteers, aged between 18 and 72 years, who present total loss of sensation and movement and whose injuries occurred in the last 72 hours. The substance will be applied directly to the spinal cord. The initial phase will last at least six months.
If the results are favorable, the product will move on to phases 2 and 3, focused on demonstrating effectiveness. The substance is produced from a protein extracted from the placenta and talks are ongoing with the Ministry of Health about a possible future offering through the Sistema Único de Saúde (SUS), Brazil’s public health system, should the drug be approved.
Read the article in the original language.