CAVUX FFS-LX Achieved 96% Fusion Fee Verified by Impartial Core Imaging Lab in Latest Scientific Examine
PLEASANTON, Calif., Jan. 11, 2024 /PRNewswire/ — Windfall Medical Expertise, Inc. (PMT), a medical gadget innovator targeted on enhancing surgical outcomes for high-risk spine surgical procedure sufferers, introduced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® FFS-LX: Lumbar Side Fixation System to be used in lumbar spinal fusion surgical procedure.
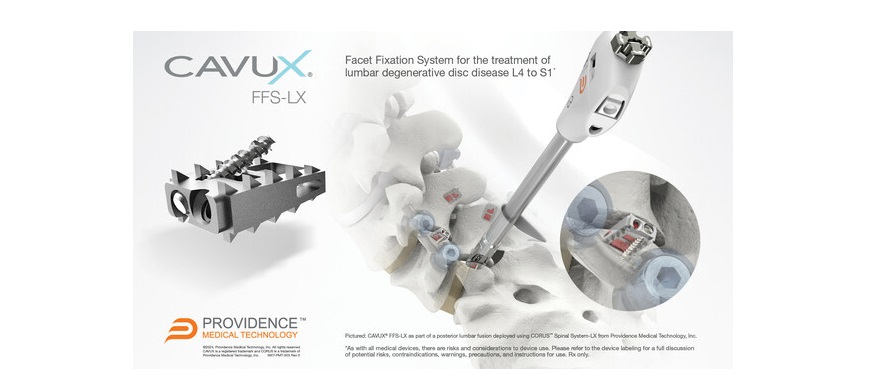
CAVUX® FFS-LX is a novel built-in cage and screw system that’s implanted bilaterally within the side joints to deal with lumbar degenerative disc illness (DDD). The implant spans the side interspace with factors of fixation at every finish of the assemble to supply extra stabilization for 1- or 2-level lumbar interbody fusion. CAVUX® FFS-LX could also be used with or with out pedicle screws and rods and is implanted utilizing the corporate’s CORUS™ Spinal System-LX tissue-sparing entry and spinal fusion system.
The clearance marks the corporate’s enlargement into the $2 billion lumbar spine market characterised by the next surgical failure fee than within the cervical spine. Sufferers who fail to fuse after a lumbar spine fusion surgical procedure face a considerable danger of added issues, struggling, and expensive revision procedures. CAVUX® FFS-LX was designed to supply elevated stabilization following lumbar fusion procedures to be able to enhance fusion charges and cut back future issues and reoperations.
The clearance was supported by medical research information demonstrating a powerful security and efficacy profile. Abstract medical research information reviewed by the FDA in contemplating the clearance included:
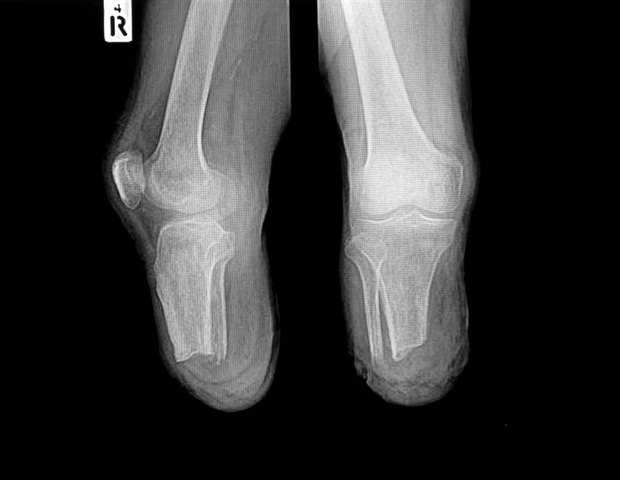
- 57 sufferers had been evaluated with a median follow-up of 30 months. The median age was 45, the median physique mass index was 30, and 68% of topics reported nicotine use as a danger issue.
- 96% of ranges had been decided to be fused as outlined by a variety of movement lower than 5° on flexion/extension x-rays assessed by an unbiased core imaging lab.
- Clinically significant enchancment in ache was achieved in 79% of topics.
“At Windfall, we’re pushed to enhance medical outcomes and stop surgical failures for high-risk sufferers,” commented Jeff Smith, Co-founder and CEO of Windfall Medical Expertise. “CAVUX® FFS-LX builds off our profitable cervical platform and applies the identical ideas to the lumbar spine the place the problem of treating high-risk sufferers is much more pronounced. We’re excited to be launching into the lumbar market and supply high-risk sufferers a brand new possibility for a fusion success.”
Joseph O’Brien, MD, a number one orthopedic spine surgeon specializing in minimally invasive spine surgical procedure, and founding father of the Washington Backbone and Scoliosis Institute commented, “I’m excited concerning the FDA clearance of CAVUX FFS-LX as a remedy possibility for sufferers handled with lumbar fusion for degenerative disc illness. The implant gives elevated stabilization on the lumbar side degree for minimally invasive fusion procedures. This new possibility will broaden the spine specialist’s remedy arsenal and sure result in elevated fusion charges for sufferers. I’ve seen glorious outcomes from this expertise in difficult fusion sufferers.”
For extra data and full product indications go to providencemt.com/products-cavux-ffs-lx/
About Windfall Medical Expertise, Inc.
Windfall Medical Expertise, Inc. is a number one medical gadget firm targeted on advancing spine surgical procedure applied sciences. Its dedication to enhancing medical outcomes and lowering failures in high-risk spinal surgical procedure has led PMT to be on the forefront of growing progressive options like CAVUX® FFS-LX. The corporate’s complete vary of merchandise, together with CORUS™ Spinal Programs, CAVUX® Side Fixation Programs, and ALLY® Bone Screws and Side Screws underscores its dedication to excellence in spinal care.
SOURCE Windfall Medical Expertise, Inc.






















Discussion about this post