Summary: Researchers have identified how a single mutation in the GPX4 enzyme causes neurons to undergo ferroptosis, leading to severe early-onset dementia. The study provides the first molecular evidence that ferroptosis can directly drive neurodegeneration in the human brain.
By disrupting a small “fin-like” loop that anchors GPX4 to neuronal membranes, the mutation prevents detoxification of lipid peroxides, allowing oxidative damage to spread. Early experiments show that blocking ferroptosis can slow cell death, pointing to new therapeutic directions for childhood dementia and potentially broader neurodegenerative diseases.
Key facts
Critical enzyme identified: a mutation in GPX4 alters its membrane anchoring loop, preventing the detoxification of lipid peroxides. Ferroptosis drives degeneration: This is the first molecular evidence that ferroptosis directly causes neurodegeneration in the human brain. Therapeutic potential: Ferroptosis-blocking compounds slowed neuronal death in early experiments, offering new avenues for treatment.
Source: Helmholtz
Why do neurons die in dementia? Can this process be slowed down?
An international team led by Prof. Marcus Conrad, director of the Institute of Metabolism and Cell Death at Helmholtz Munich and professor of Translational Redox Biology at the Technical University of Munich (TUM), now describes in Cell how neurons protect themselves against ferroptotic cell death.
A central element of this defense mechanism is the selenoenzyme glutathione peroxidase 4 (GPX4). A single mutation in the gene encoding GPX4 can alter a crucial and previously unknown component of the enzyme’s function. In affected children, this leads to severe early-onset dementia.
When fully functional, GPX4 inserts a short protein loop (a sort of “fin”) into the inner side of the neuronal cell membrane, allowing the enzyme to neutralize harmful substances known as lipid peroxides.
Navigating along the cell membrane
“GPX4 is a bit like a surfboard,” says Conrad. “With its fin immersed in the cell membrane, it sweeps along the inner surface and rapidly detoxifies lipid peroxides as it goes.”
A single point mutation found in children with early-onset dementia disrupts this fin-shaped protein loop: the enzyme can no longer properly insert into the membrane to perform its cellular protective function. Lipid peroxides are free to damage the membrane, causing ferroptosis and cell rupture, and neurons die.
The study began with three children in the United States who suffer from an extremely rare form of early childhood dementia. All three carry the same change in the GPX4 gene, known as the R152H mutation.
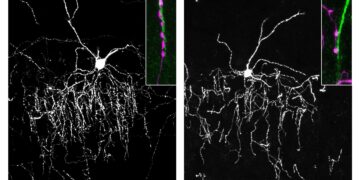
Using cell samples from an affected child, the researchers were able to study the effects of the mutation in more detail and reprogrammed the cells back to a stem cell-like state. From these reprogrammed stem cells, they generated cortical neurons and three-dimensional tissue structures that resemble primitive brain tissue: so-called brain organoids.
Laboratory evidence confirms: without functional GPX4, dementia develops
To understand what happens at the whole-organism level, the team introduced the R152H mutation into a mouse model, thereby specifically altering the GPX4 enzyme in different types of nerve cells.
As a result of impaired GPX4 function, the animals gradually developed severe motor deficits, with dying neurons in the cerebral cortex and cerebellum, and pronounced neuroinflammatory responses in the brain, a pattern that closely mirrors observations in affected children and closely resembles the profiles of neurodegenerative diseases.
In parallel, the researchers analyzed which proteins change in abundance in the experimental model. They observed a pattern strikingly similar to that seen in patients with Alzheimer’s disease: numerous proteins that are upregulated or downregulated in Alzheimer’s disease were also dysregulated in mice that lacked functional GPX4. This suggests that ferroptotic stress may play a role not only in this rare early-onset disorder, but also potentially in more common forms of dementia.
A new view on the causes of dementia
“Our data indicate that ferroptosis may be a driving force behind neuronal death, not just a side effect,” says Dr. Svenja Lorenz, one of the first authors of the study.
“Until now, dementia research has often focused on protein deposits in the brain, so-called amyloid-β plaques. We are now putting more emphasis on the damage to cell membranes that sets this degeneration in motion in the first place.”
Initial experiments also show that cell death caused by loss of GPX4 can be slowed in cell culture and in mouse models using compounds that specifically inhibit ferroptosis.
“This is an important proof of principle, but it is not yet a therapy,” says Dr. Tobias Seibt, a nephrologist at LMU University Hospital in Munich and co-author of the study.
Dr. Adam Wahida, also first author of the study, adds: “In the long term, we can imagine genetic or molecular strategies to stabilize this protective system. However, for now, our work clearly remains in the realm of basic research.”
Basic research helps understand the roots of diseases
The study is the result of a research network that has grown over many years bringing together genetics, structural biology, stem cell research and neuroscience, with several dozen scientists at multiple sites around the world.
“It has taken us almost 14 years to link a small, yet unrecognized structural element of a single enzyme to a serious human disease,” says Marcus Conrad.
“Projects like this vividly demonstrate why we need long-term funding for basic research and international multidisciplinary teams if we are to truly understand complex diseases such as dementia and other neurodegenerative diseases.”
Key questions answered:
A: The mutation alters a small structural loop necessary for GPX4 to adhere to neuronal membranes, preventing it from neutralizing lipid peroxides and allowing ferroptosis to kill neurons.
A: It provides the first direct molecular evidence that ferroptosis not only correlates with neurodegeneration, but may also actively drive neuronal death in the human brain.
A: Early experiments show that inhibiting ferroptosis slows neuronal loss, suggesting it may become a future strategy for childhood dementia and possibly more common neurodegenerative diseases.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this research news on genetics and childhood dementia
Author: Céline Gravot-Schüppel
Source: Helmholtz
Contact: Céline Gravot-Schüppel – Helmholtz
Image: Image is credited to Neuroscience News.
Original research: Open access.
“A fin-loop-like structure in GPX4 underlies neuroprotection against ferroptosis” by Marcus Conrad et al. Cell
Abstract
A fin-loop-like structure in GPX4 underlies neuroprotection against ferroptosis
Ferroptosis, driven by uncontrolled peroxidation of membrane phospholipids, differs from other modes of cell death because it lacks an initiating signal and is guarded by endogenous antioxidant defenses.
Glutathione peroxidase 4 (GPX4) is the gatekeeper of ferroptosis, although its membrane protective function remains poorly understood.
Here, structural and functional analyzes of a missense mutation in GPX4 (p.R152H), which causes early-onset neurodegeneration, revealed that this variant alters membrane anchoring without significantly affecting its catalytic activity.
Spatiotemporal deletion of Gpx4 or neuron-specific expression of GPX4R152H in mice induced degeneration of cortical and cerebellar neurons, accompanied by progressive neuroinflammation.
Cortical neurons and forebrain organoids derived from patient-induced pluripotent stem cells (iPSCs) showed increased ferroptotic vulnerability, reflecting key pathological features, and were sensitive to inhibition of ferroptosis. Neuroproteomics revealed Alzheimer’s-like signatures in affected brains.
These findings highlight the need for proper GPX4 membrane anchoring, establish ferroptosis as a key driver of neurodegeneration, and provide the rationale for targeting ferroptosis as a therapeutic strategy in neurodegenerative diseases.