Carlsbad, CA, March 10, 2025 (Globe Newswire) – Aurora Spine Corporation (“Aurora Spine” or “Company”) (TSXV:ASG) (OTCQB:ASAPF) has announced the completion of patients who have announced the completion of the intervention for the intervention today. Suffering from lower back pain due to symptomatic degenerative disc disease. This prospective, multicenter, multispecialized clinical trial is the first of its kind to evaluate the safety and efficacy of lumbar intervention fusion devices, and represents an important milestone in advances in spinal cord care.
Purification studies designed to bridge the gap between conservative treatment and more invasive surgical options successfully enrolled a complete cohort of patients across multiple US centers. Data from this pioneering study were published over a year, followed by two years of cohort publication, providing robust, long-term insight into patient outcomes. The results were presented at the American Association for Pain and Neuroscience (ASPN) Annual Conference in July 2025, highlighting Aurora Spine’s commitment to evidence-based innovation.
“We’ve seen a lot of different types of neurosurgery,” said Dr. Stephen Fallowski, a fellow neurosurgery student in Lancaster, Pennsylvania. “The data we collect is important and is the foundation for improving our patients’ lives, not just numbers. The Refine Study provides the highest level of scientific evidence to guide clinical decision-making and enhance standard of care.”
Dr. Stephen Anagnost, an orthopedic surgeon in spine and musculoskeletal medicine in Oklahoma, reflected this optimism. “The impact of purification studies cannot be overstated. As an orthopedic surgeon, I see first-hand the need for reliable, minimally invasive solutions. This comprehensive multispecialist approach in this trial shapes the future of spinal fusion and provides patients with safer and more effective options supported by robust data.”
Dr. Jason Pope, a pain doctor at the Evolve Restorative Center in Santa Rosa, California, highlighted the potential for transformation in this study. The Refine Study shows how Aurora Spine pushes boundaries, providing hope and concrete improvements for those suffering from back pain. ”
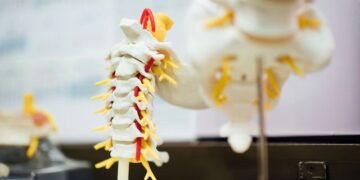
Aurora Spine’s Refine Study stands out by its co-design, uniting neurosurgeons, orthopedic surgeons, and interventional pain physicians to assess the ZIP™ Misintermediated Fusion System. This minimally invasive technique aims to address unmet needs in the treatment of lumbar spinal stenosis and degenerative disc disease, providing patients with a less invasive alternative to traditional spinal surgery.
“We are extremely proud to reach this milestone,” said Trent Northcutt, president and CEO of Aurora Spine. “Completing our enrolment with Refine Research highlights our commitment to advancing spinal health through cutting-edge technology and rigorous clinical research. We look forward to continuing to promote life-changing innovations by sharing our results with ASPN’s medical community in July.”
The company expects the Refine Study findings will enhance the effectiveness and safety of the ZIP™ system and further solidify the Aurora Spine’s position as a trail blazer in the spine implant market. The Aurora spine remains committed to empowering physicians and improving patient outcomes through groundbreaking clinical evidence and minimally invasive solutions.
About Aurora Spine
Aurora Spine focuses on bringing new solutions to the spinal implant market through a series of innovative, minimally invasive regenerative spinal implant technologies. Additional information can be accessed at www.aurora-spine.com or www.aurorapaincare.com.
TSX Venture Exchange also does not accept responsibility for the validity or accuracy of this release, as defined in the TSX Venture Exchange policy.
Forward-looking statements
This news release contains forward-looking information with most known unknown risks and uncertainties that go beyond control of the Aurora spine, including those listed in “Risk Factors” and “Forward-Looking Information Notes” in the Aurora Spine Final Prospectus (collectively “Advanced Information”) and those listed in “Risk Factors” and “Forward-Looking Information Notes.” The forward-looking information in this news release includes information regarding the use and success of the company’s products in surgical procedures. Aurora Spine is warning investors of Aurora Spine securities about important factors that could cause the actual results of Aurora Spine to differ materially from the forward-looking statements contained in this news release. Any expectations, beliefs, plans, objectives, purposes, assumptions, or statements that represent or involve discussions regarding future events or future events or performance are forward-looking, not historical facts and may contain estimates, assumptions, assumptions, and uncertainties that may cause results or results that differ unilaterally from those expressed in such forward-looking statements. We cannot guarantee that the expectations listed here will be proven correct. Therefore, future investors should not place undue confidence in these forward-looking statements. These statements are only spoken at the time of this press release and Aurora Spine is not obligated to update or modify them to reflect new events or circumstances.
contact:
Aurora Spine Corporation
Trent Northcut
President and CEO
(760) 424-2004
Chad Kraus
CFO
(760) 424-2004
www.aurora-spine.com