Summary: A new study shows that precisely manipulating brain activity during sleep can help mice retain memories that would normally fade, offering a potential avenue for treating memory loss conditions. The researchers identified a specific sleep-related pattern (large sharp waves) that signals when new experiences are transferred from the hippocampus to the neocortex for long-term storage.
By boosting these waves at just the right moment through optogenetics, the scientists allowed the mice to remember brief encounters that they would normally forget. Even mice engineered with cognitive disabilities showed restored memory consolidation.
Since sleep-dependent memory mechanisms are highly conserved in mammals, the findings may open new doors for addressing memory decline in conditions such as Alzheimer’s disease.
Key facts
Sleep waves drive memory: Large, sharp waves during sleep act as markers of new experiences that are transferred to long-term storage. Optogenetically enhanced recall: Stimulating neurons at the peaks of the waves allowed mice to remember events that would otherwise be forgotten. Therapeutic potential: The approach restored memory consolidation in cognitively impaired mice, highlighting relevance for dementia research.
Source: Cornell University
Manipulating the mice’s brains during sleep improved their ability to remember new experiences that would normally be forgotten, a finding with important implications for the treatment of Alzheimer’s disease and other forms of dementia that act on similar processes.
The study published in Neuron is relevant to humans, since the basic mechanisms of memory formation are very similar among mammals.
By selectively manipulating brain activity at specific times during sleep, the scientists found that the mice remembered new experiences that would otherwise be too brief to retain.
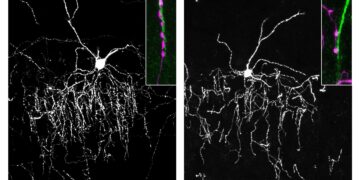
The researchers identified large sharp waves, a subset of brain activity patterns about 100 milliseconds long that are involved in consolidating and transferring new experiences from the hippocampus to the neocortex, where they are stored more permanently. The large sharp waves can now help researchers identify when new experiences are being converted into long-term memory.
“This study advances our understanding of memory processing in the brain,” said Azahara Oliva, assistant professor of neurobiology and behavior. Oliva is the lead author of the study along with assistant professor Antonio Fernández-Ruiz.
In the study, Fernández-Ruiz and Oliva recorded neuronal activity in the hippocampus and neocortex. They identified large sharp waves in the hippocampus, which occur during sleep and then propagate to the neocortex.
“The waves are mediating the transfer of memory from initial encoding in the hippocampus to stable long-term storage in the neocortex,” Fernández-Ruiz said.
The researchers observed that when an animal did not remember an experience, the large sharp waves were weak during sleep, but when it did remember, there were many of these waves.
Once the researchers identified this pattern, they used an advanced technique called optogenetics to illuminate the brain with an optical fiber that then selectively activated neurons.
Optogenetics allowed the team to stimulate neurons at precise times to drive large sharp waves, which then cemented the memory of an event the animal experienced just before sleep.
The team exposed mice to a new toy for five minutes and tested it four hours later and found that the animals did not remember. They then increased the waves associated with that experience during sleep, and found that the mouse did remember the object. The technique even worked in mice engineered to have cognitive deficits.
“We were able to extend this memory consolidation in a condition where the animals cannot remember without our help,” Fernández-Ruiz said.
This has important implications for better understanding Alzheimer’s disease, as these memory consolidation processes are also altered in humans. In next steps, the researchers will apply the same manipulations in mice engineered to exhibit Alzheimer’s-like conditions.
Key questions answered:
A: Large sharp waves (short bursts of coordinated neural activity) signal the conversion of new experiences into long-term memories.
A: By timing optogenetic stimulation to coincide with these waves, they boosted memory consolidation during sleep.
A: Memory consolidation is impaired in disorders such as Alzheimer’s, and improving these sleep-based processes could guide future treatments.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this research news on sleep and memory.
Author: Becka Bowyer
Source: Cornell University
Contact: Becka Bowyer – Cornell University
Image: Image is credited to Neuroscience News.
Original research: Open access.
“Large sharp waves promote the reactivation and consolidation of hippocampo-cortical memory during sleep” by Antonio Fernández-Ruiz et al. Neuron
Abstract
Large sharp waves promote reactivation and consolidation of hippocampo-cortical memory during sleep
During sleep, joint activity patterns that encode recent experiences are reactivated in the hippocampus and cortex.
This reactivation is coordinated by hippocampal sharp waves (SWR) and is thought to support the early stages of memory consolidation.
However, only a minority of sleep ROEs are associated with memory reactivation in the hippocampus and its posterior areas. It is unknown whether that subset of SWR has specific physiological characteristics and directly contributes to memory performance.
We identified a specific subset of large SWRs linked to memory reactivation in both the hippocampus and prefrontal cortex (PFC) of mice, and found that their occurrence was selectively increased during sleep following new learning.
Increasing closed-loop optogenetic SWR during sleep was sufficient to enhance joint memory reactivation in the hippocampus and PFC.
This manipulation also improved subsequent memory retrieval and hippocampal-PFC coordination during wakefulness, causally linking both phenomena to SWR-associated ensemble reactivation during sleep.