Summary: New findings show that deletion of Centaurin-α1, a protein elevated in Alzheimer’s disease, significantly reduces inflammation, plaque accumulation and cognitive deficits in a well-established mouse model. Deleting this protein normalized several brain pathways, protected neural connections in the hippocampus, and improved spatial learning.
Although plaque reduction varied across brain regions, the overall improvements point to Centaurin-α1 as a potential therapeutic target. The researchers now aim to determine whether reducing the protein in adulthood could also slow the progression of the disease.
Key facts
Protein knockdown, damage reduction: Centaurin-α1 knockdown reduced neuroinflammation and reduced hippocampal plaque burden by approximately 40%. Cognitive benefits: Mice lacking the protein showed better preservation of neural connections and better spatial learning. Therapeutic potential: Normalization of gene expression patterns suggests that Centaurin-α1 may regulate multiple processes related to Alzheimer’s.
Source: Max Planck Institute
New research published in the journal eNeuro examined whether removing a protein elevated in the brains of people with Alzheimer’s could prevent or reduce damage and behavioral symptoms in a mouse model of Alzheimer’s disease.
“Previous work by our research team and others found evidence that a specific protein called Centaurin-α1 is involved in the progression of Alzheimer’s damage within neurons,” explained the study’s lead author, Dr. Erzsebet Szatmari.
“To confirm the role of this protein and see if it could be a good therapeutic target, we tested whether genetically knocking it out would prevent or slow disease progression in a mouse model.”
The scientists used a well-characterized model of Alzheimer’s disease in mice. The disease model (called J20) contains two genetic mutations associated with rare familial variants of Alzheimer’s disease.
These animals develop brain tissue changes and behavioral deficits characteristic of many symptoms seen in human Alzheimer’s disease, including neuroinflammation, accumulation of neuronal plaques, loss of synapses, and deficits in spatial memory and learning.
Deletion of Centaurin-α1 decreases disease damage
The research team genetically deleted the Centaurin-α1 protein from Alzheimer’s model mice to investigate whether this would alter the development of the disease’s symptoms.
Some of the earliest deficits observed in the disease model include neuroinflammation. While Alzheimer’s model mice showed widespread markers of neuroinflammation, those lacking Centaurin-α1 did not.
Knockdown of Centaurin-α1 also reduced amyloid plaque formation, a hallmark of Alzheimer’s disease. In the hippocampus, a highly affected area of the brain, the plaques were reduced by approximately 40%.
However, this reduction was not observed in the neocortex, suggesting that plaque formation in the disease may differ between brain regions and that treatments to prevent its accumulation may need to be multiple.
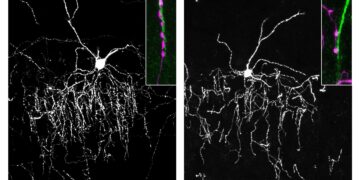
In addition to improving neuroinflammation and plaque accumulation, the researchers found that deletion of Centaurin-α1 partially protected against the loss of neuronal connections in the hippocampus, critical for spatial learning.
This finding suggested that the impaired spatial learning characteristics observed in the disease model may also be improved by deleting Centaurin-α1. Indeed, deletion of Centaurin-α1 improved spatial learning deficits in mice.
Centaurin-α1 as a possible therapeutic target
“We were encouraged by the behavioral changes observed in Alzheimer’s model mice lacking Centaurin-α1, confirming that the protein contributes to the progression of cognitive symptoms and could therefore be a valuable therapeutic target. However, we still have a lot to learn about how it works in the brain to worsen the disease,” Szatmari said.
To begin to understand how Centaurin-α1 might influence disease progression, the research team compared the brain composition of healthy mice, disease model mice, and disease model mice without Centaurin-α1 using gene expression analysis.
As expected, many components of the brain tissue of the disease model mice were altered, with some components increased and others decreased.
However, disease model mice lacking Centaurin-α1 showed a somewhat normalized brain composition, with components that had been increased decreasing and those that had been reduced increasing.
“We believe that Centaurin-α1 may play a multifunctional role in regulating signaling processes in the brain that alter gene expression and the composition of many molecules. This aberrant signaling may ameliorate disease progression and symptoms through metabolic deficits, neuroinflammation, amyloid processing, and dysfunction of neuronal connections,” describes senior author and scientific director of the MPFI, Dr. Ryohei Yasuda.
“Although more research is needed to determine whether depletion of Centaurin-α1 can benefit the human brain, the evidence so far suggests that Centaurin-α1 is a promising candidate for future therapeutic development.”
The team is advancing the investigation of Centaurin-α1 as a potent regulator of multiple processes related to Alzheimer’s and whether reducing its activity in adulthood, rather than eliminating it from birth, could also slow the progression of the disease.
Recently, they found that loss of Centaurin-α1 reduced symptoms in a mouse model of another disease, multiple sclerosis (MS), suggesting that its role in disease progression may extend to multiple neurodegenerative diseases.
Funding: This work was funded by the BrightFocus Foundation, the 54 Palm Beaches Community Foundation, NIA, the Max Planck Foundation, ECU Startup funds, and ECU URCA awards. This content is the sole responsibility of the authors and does not necessarily represent the official views of the funders.
Key questions answered:
A: Neuroinflammation decreases, amyloid plaques fall in the hippocampus, neuronal connections are better preserved, and spatial learning improves.
A: Removing it normalizes altered gene expression patterns and reduces multiple Alzheimer’s-related problems, suggesting it influences multiple disease pathways.
A: No: plaques decreased significantly in the hippocampus but not in the neocortex, indicating region-specific mechanisms and the possible need for multiple therapies.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this news about Alzheimer’s research
Author: Lesley Colgan
Source: Max Planck Institute
Contact: Lesley Colgan – Max Planck Institute
Image: Image is credited to Neuroscience News.
Original Research: Closed access.
“Lack of ADAP1/Centaurin-α1 improves cognitive impairment and neuropathological features in a mouse model of Alzheimer’s disease” by Erzsebet Szatmari et al. eNeuro
Abstract
Lack of ADAP1/Centaurin-α1 improves cognitive impairment and neuropathological features in a mouse model of Alzheimer’s disease
ArfGAP, with dual PH domain-containing protein 1/Centaurin-α1 (ADAP1/CentA1), is a highly conserved Ras-anchoring and Arf6 GTPase-activating protein enriched in the brain. CentA1 participates in dendritic growth and arborization, synaptogenesis, and axonal polarization by regulating actin cytoskeleton dynamics.
The upregulation of CentA1 and its association with amyloid plaques in human Alzheimer’s disease (AD) brain suggest the role of this protein in AD progression.
To understand the role of CentA1 in neurodegeneration, we crossed CentA1 knockout (KO) mice with the J20 mouse model of AD. We evaluated AD-associated behavioral and neuropathological characteristics and gene expression profiles in J20 and J20 crossed with male CentA1 KO (J20xKO) mice to determine the impact of knockdown of CentA1 expression on AD-related phenotypes.
Spatial memory assessed by the Morris water maze test showed a significant impairment in J20 mice, which was rescued in J20xKO mice. Furthermore, neuropathological features of AD, such as amyloid plaque deposits and neuroinflammation, were significantly reduced in J20xKO mice.
To identify potential mediators of rescue of the AD phenotype, we analyzed genes differentially expressed between genotypes. We found that the changes in the genetic profile by CentA1 deletion from J20 (J20xKO vs J20) were anticorrelated with the changes caused by APP overexpression (J20 vs wild type), consistent with the rescue of J20 phenotypes by CentA1 KO.
In summary, our data indicate that CentA1 is required for the progression of AD phenotypes in this model and that targeting CentA1 signaling could have therapeutic potential for the prevention or treatment of AD.