Cedars-Sinai researchers have discovered a healing mechanism that could one day be harnessed to help treat patients with spinal cord injuries, strokes and neurological conditions such as multiple sclerosis. His study, published in Nature, describes a previously unknown function of astrocytes, a type of cell in the central nervous system.
“Astrocytes respond critically to diseases and disorders of the central nervous system—the brain and spinal cord,” said neuroscientist Joshua Burda, PhD, assistant professor of Biomedical Sciences and Neurology at Cedars-Sinai and senior author of the study. “We found that astrocytes far from the site of an injury actually help drive spinal cord repair. Our research also uncovered a mechanism used by these unique astrocytes to tell the immune system to clean up debris resulting from injury, which is a critical step in the tissue healing process.”
The researchers called these astrocytes “remotely injured astrocytes,” or LRA, and identified several distinct subtypes of LRA. Their study describes for the first time how an AKI subtype remotely senses and responds to tissue injury.
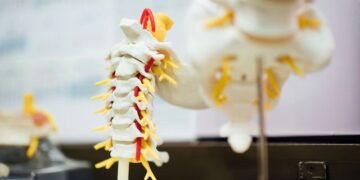
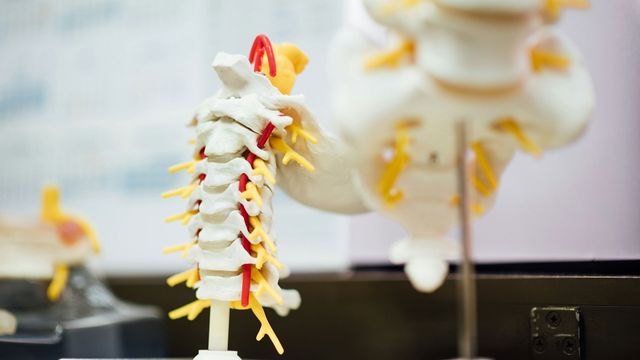
The spinal cord is a collection of nervous tissue that runs from the brain to the back. At its center is gray matter, which contains the bodies of nerve cells and supporting cells called astrocytes. Surrounding it is white matter, made up of astrocytes and long nerve fibers that extend up and down the spinal cord to send signals between the brain and the rest of the body. Astrocytes help keep the nervous system healthy and ensure these signals flow smoothly.
Spinal cord injuries damage nerve fibers, paralyze parts of the body, and disrupt sensory information such as touch and temperature. The cut fibers die and become debris. In most other types of tissue in the body, inflammation occurs only at the site of injury. But because of the length of the nerve fibers in the spinal cord, damage and inflammation extend far beyond the site of injury.
Researchers looked at laboratory mice with spinal cord injury and found that LRAs play an important role in supporting nervous system repair. They saw strong evidence of the same mechanism in tissue samples from human patients with spinal cord injury.
The Burda Laboratory identified a subtype of AKI that sends a protein called CCN1 to signal immune cells called microglia.
“One function of microglia is to serve as primary garbage collectors in the central nervous system,” Burda said. “After tissue damage, they eat pieces of nerve fiber remains, which are very fatty and can cause a kind of indigestion. Our experiments showed that the astrocyte CCN1 tells the microglia to change their metabolism so that they can better digest all that fat.”
Burda said this efficient debris clearing could play a role in the spontaneous recovery found in many spinal cord injury patients. In the absence of the astrocyte-derived protein CCN1, the researchers found that recovery is drastically impaired.
“If we eliminate the CCN1 astrocyte, the microglia eat, but do not digest. They call more microglia, which also eat but do not digest,” Burda said. “Large clumps of debris-filled microglia form, which increases inflammation along the spinal cord. And when that happens, the tissue doesn’t repair itself as well.”
When researchers looked at spinal cord tissue from human patients with multiple sclerosis, they found the same mechanism at work, Burda said. He added that these fundamental principles of tissue repair likely apply to any type of brain or spinal cord injury.
“The role of astrocytes in central nervous system healing is very understudied,” said David Underhill, PhD, chair of the Department of Biomedical Sciences. “This work strongly suggests that remotely injured astrocytes offer a viable pathway to limit chronic inflammation, enhance functionally significant regeneration, and promote neurological recovery after brain and spinal cord injury and in disease.”
Burda is now leading efforts to harness this CCN1 mechanism in spinal cord healing and to further investigate the role of astrocyte CCN1 in inflammatory neurodegenerative diseases and aging.
Reference: McCallum S, Suresh KB, Islam TS, et al. Astrocytes remote from the lesion govern microglia-mediated white matter repair. Nature. 2025. doi: 10.1038/s41586-025-09887-y
This article has been republished from the following materials. Note: Material may have been edited for length and content. For more information, contact the cited source. You can access our press release publishing policy here.