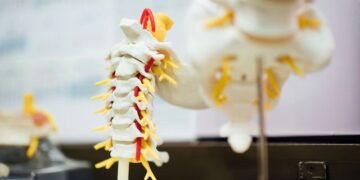
Recent notices of allowances are for Aclarion’s 24th issued US patent propionic acid (PA), which may suggest that the literature suggests a bacterial infection; Disc patents may indicate pain in disc patents. To identify potential infections and pain throughout the body
Bloomfield, Colorado, February 11, 2025 (Globe Newswire) – Aclarion, Inc. , (“Aclarion” or “Company”) (NASDAQ: ACON, ACONW), an algorithm that helps doctors, a healthcare technology company that utilizes biomarkers, locate chronic low back pain, today announced the US On the 24th, when the patent was issued, it was announced that it had received a notice of allowance from the US Patent and Trademark Office (USPTO). A patent previously issued in the Aclarion portfolio limits the use of propionic acid (PA) identification by magnetic resonance spectroscopy (MRS) to DISC, but this new patent uses Aclarion’s MRS to make its whole body Expand the ability to identify PAs and identify potential sources of infection. And pain.
“Propionate is a normal human physiological metabolite, but there are many known side effects and disorders associated with propionate,” said Dr. Jeff Lotz, vice-chairman at University of California, San Francisco. “Aclarion is a global leader in harnessing the power of MR spectroscopy, and with this recent addition of the patent, Aclarion commercializes the use of MRS beyond low back pain, allowing the identification and measurement of propionic acid throughout the body. It further expands the possibilities of inclusion. Its meaning is profound.”
MRS is the existing capabilities of the major magnetic resonance imaging (MRI) scanners currently commonly used in medical imaging. MRI creates images of the body’s soft tissue structure, and MRS generates spectral data information regarding the levels of various chemical biomarkers within soft tissues, such as proteins, carbohydrates, and acids. However, traditional MRS post-processing approaches are challenging and unable to generate sufficient quality data for reliable chemical analysis. Some of Aclarion’s patents are related to new inventions that address many of these challenges, bringing more reliable, high-quality spectral data for more sophisticated chemical biomarker analysis, and the Nociscan solution and ensure the accuracy and reliability of other MRS applications.
To find the Nociscan Center, check out the site map here.
Aclarion, Inc. About
Aclarion is a healthcare technology company that leverages magnetic resonance spectroscopy (“MRS”), proprietary signal processing technology, biomarkers, and extended intelligence algorithms to optimize clinical treatments. The company will help doctors distinguish between Nociscan, the SaaS platform supported by the first evidence, from the non-unstable discs with lumbar pain and non-unstable discs. Through cloud connections, Nociscan receives magnetic resonance spectroscopy (MRS) data from MRI machines for each lumbar disc being evaluated. In the cloud, proprietary signal processing techniques extract and quantify chemical biomarkers that have been proven to be related to disc pain. The biomarker data is entered into a proprietary algorithm to indicate whether the disk is the cause of the pain. When used with other diagnostic tools, Nociscan provides important insight into the patient’s lower back pain location, and doctors clarify to optimize treatment strategies. For more information, please visit www.aclarion.com.
This press release is for informational purposes only and does not intend to offer or solicit or recommend an offer or solicitation of a sale or offer to sell, or solicit a power of attorney, consent, voting or approval. Aclarion, Inc. securities. Before registration or qualification under the securities law of such jurisdiction, in any jurisdiction, such offer, sale, issuance, or transfer is illegal, prior to registration or qualification under such jurisdiction, No transfer will be made.
Forward-looking statements
This press release includes future outcomes, performance, prospects and opportunities within the meaning of Section 27A of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements that are not historical facts such as “anticipation,” “believe,” “expectation,” or similar expressions are forward-looking statements. These forward-looking statements are based on current plans and expectations of management, and many uncertainties and risks that could significantly affect our current plans and expectations, as well as future operations results. and is exposed to financial position results. These and other risks and uncertainties have been discussed more fully in their filings with the Securities and Exchange Commission. Readers will be entitled “Risk Factors” in our annual report on Form 10-K for the year ended December 31, 2023, as well as in the prospectus and subsequent filings with the Securities and Exchange Commission. We recommend that you review any other disclosures included. . Forward-looking statements contained in this announcement will be made as of this date and the Company shall not be subject to any obligation to publicly update or amend any forward-looking statements as a result of new information, future events, or other results. I don’t have any responsibility.
Investor Contact Information:
Giraffe M. Smith
PCG Advisory, Inc.
646.823.8656
ksmith@pcgadvisory.com
Media Contact:
Jenny Kim
SPRIG Consulting
jennie@sprigconsulting.com