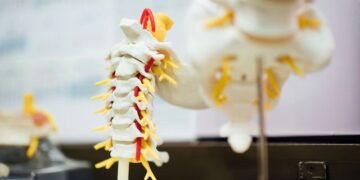
Spinal deformities such as scoliosis and kyphosis are among the most serious complications of neurofibromatosis type 1 (NF1), a genetic disorder that affects approximately one in 3,000 people. These deformities often begin in childhood, worsen rapidly, and can lead to chronic pain, reduced mobility, and may require major surgery. Despite its frequency and severity, there are currently no approved drug treatments to prevent NF1-related spinal deformity.
In a new study published in Volume 13, Issue 103 of the journal Bone Research on December 16, 2025, a research team led by Dr. Céline Colnot from Univ Paris Est Creteil in France set out to identify the cellular origin and biological mechanism behind this condition. They used a genetically engineered mouse model, known as the Prss56-Nf1 knockout mouse, that reproduces multiple symptoms of NF1, including tumors, bone abnormalities, and spinal curvature.
Using high-resolution micro-CT imaging, the researchers showed that the mice developed progressive spinal deformities beginning in adulthood, with vertebral changes closely resembling those seen in NF1 patients. By genetically marking the affected cells, the team traced the abnormalities to an unexpected source: bone-forming osteoblasts that carry mutations in the NF1 gene.
“Our data show that NF1-deficient osteoblasts accumulate in the vertebrae and are directly related to the severity of spinal curvature,” says Dr. Colnot. “The more mutant bone cells are present, the more pronounced the deformity becomes.”
Additional analyzes revealed that these osteoblasts remain locked in an abnormally active state due to persistent activation of the RAS-MAPK signaling pathway, which regulates cell growth and differentiation. As a result, bone formation and resorption become unbalanced, leading to excessive and disorganized bone accumulation within the spine.
“These cells continue to proliferate and do not mature properly,” says Dr. Colnot. “Over time, this disrupts the normal architecture of the vertebrae and contributes to spinal curvature.”
Because the RAS-MAPK pathway is already the target of drugs used to treat certain NF1-related tumors, researchers tested whether blocking this signal could prevent spinal deformity. When adult mutant mice were treated with a combination of the MEK/SHP2 inhibitors selumetinib and RMC-4550, the progression of spinal deformities was stopped.
“Pharmacological inhibition of this pathway prevented spinal deformity in our model,” the authors note, highlighting the potential of repurposing existing drugs for NF1-related skeletal disease.
The researchers caution that their findings are limited to mice and that more studies are needed to determine whether similar mechanisms drive spinal deformity in people with NF1. Still, their work offers hope for understanding and eventually treating a condition that currently leaves patients with few options beyond monitoring and surgery.
Fountain:
Magazine reference:
Kovaci, F., et al. (2025). Pharmacological inhibition of the RAS pathway alleviates spinal deformity in a mouse model of neurofibromatosis type 1. Bone Research. doi:10.1038/s41413-025-00492-3. https://www.nature.com/articles/s41413-025-00492-3