Summary: A new study challenges the long-held belief that Alzheimer’s disease cannot be reversed. The researchers showed that a severe drop in NAD+, a central energy molecule, drives Alzheimer’s pathology in both human brains and mouse models.
Restoring the proper NAD+ balance with the drug P7C3-A20 not only prevented disease in at-risk mice, but also reversed advanced pathology, repairing brain damage and fully restoring cognitive function. The findings point to a major shift in the way Alzheimer’s can be treated, suggesting that recovery, and not just slowing decline, could one day be achieved.
Key facts
Central NAD+ Failure: Human and mouse brains with Alzheimer’s showed a dramatic loss of NAD+, affecting essential cellular functions. Reversal achieved: Restoration of NAD+ balance repaired the pathology and cognition was fully recovered even in mice with advanced disease. New treatment avenue: Targeted drug P7C3-A20 restored healthy NAD+ levels without the dangers associated with over-the-counter NAD+ boosters.
Source: University Hospital Cleveland Medical Center
For more than a century, Alzheimer’s disease (AD) has been considered irreversible. Consequently, research has focused on preventing or slowing the disease, rather than recovery.
Despite billions of dollars spent on decades of research, there has never been a clinical trial of an AD drug aimed at reversing the disease and regaining function.
Now, a research team from University Hospitals, Case Western Reserve University, and the Louis Stokes Cleveland VA Medical Center has challenged this long-standing dogma in the field. They tested whether brains already suffering from advanced AD could recover.
The study, led by Kalyani Chaubey, PhD, of the Pieper Laboratory, was published today in Cell Reports Medicine.
By studying various preclinical mouse models and human brains of AD, the team demonstrated that the brain’s inability to maintain normal levels of a central cellular energy molecule, NAD+, is a major driver of AD, and that maintaining the proper balance of NAD+ can prevent and even reverse the disease.
NAD+ levels naturally decrease throughout the body, including the brain, as people age. Without a proper balance of NAD+, cells eventually become unable to execute critical processes necessary for proper function and survival.
In this study, the team showed that NAD+ depletion is even more severe in the brains of people with AD, and that this also occurs in mouse models of the disease.
Although AD is a uniquely human condition, it can be studied in the laboratory with mice that have been engineered to express genetic mutations that cause AD in people. The researchers used two of these models.
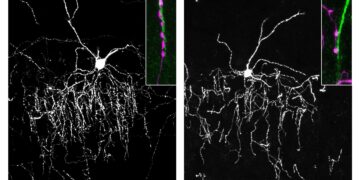
One line of mice carried multiple human mutations in amyloid processing, and the other line of mice carried a human mutation in the tau protein.
Amyloid and tau pathology are two of the major early events in AD, and both mouse lines develop AD-like brain pathology, including blood-brain barrier impairment, axonal degeneration, neuroinflammation, impaired hippocampal neurogenesis, reduced synaptic transmission, and widespread accumulation of oxidative damage.
These mice also develop severe cognitive impairments that resemble those seen in people with AD.
After discovering that brain NAD+ levels decreased precipitously in AD in both humans and mice, the research team tested whether preventing the loss of NAD+ balance in the brain before the onset of the disease, or restoring NAD+ balance in the brain after significant disease progression, could prevent or reverse AD, respectively.
The study built on their previous work, published in the Proceeding of the National Academy of Sciences USA, showing that restoring the brain’s NAD+ balance achieves pathological and functional recovery after severe and long-lasting traumatic brain injury.
They restored NAD+ balance by administering a now well-characterized pharmacological agent known as P7C3-A20, developed in Pieper’s laboratory.
Surprisingly, preserving NAD+ balance not only protected mice from developing AD, but delaying treatment in mice with advanced disease also allowed the brain to correct the major pathological events caused by the genetic mutations. Furthermore, both lines of mice completely recovered cognitive function.
This was accompanied by normalized blood levels of phosphorylated tau 217, a clinical biomarker of AD recently approved in people, confirming disease reversal and highlighting a potential biomarker for future clinical trials.
“We were very excited and encouraged by our results,” said Andrew A. Pieper, MD, PhD, lead author of the study and director of the Brain Health Medicines Center, Harrington Discovery Institute at UH.
“Restoring the brain’s energy balance achieved pathological and functional recovery in both lines of advanced Alzheimer’s mice. Seeing this effect in two very different animal models, each driven by different genetic causes, strengthens the idea that restoring the brain’s NAD+ balance could help patients recover from Alzheimer’s.”
Dr. Pieper also holds the Morley-Mather Chair in Neuropsychiatry at UH and the CWRU Rebecca E. Barchas, MD, DLFAPA, University Chair in Translational Psychiatry. He serves as a psychiatrist and researcher at the Louis Stokes VA Geriatric Research Educational and Clinical Center (GRECC).
The results spark a paradigm shift in how researchers, clinicians, and patients can think about AD treatment in the future.
“The key takeaway is a message of hope: the effects of Alzheimer’s disease may not inevitably be permanent,” Dr. Pieper said. “The damaged brain can, under some conditions, repair itself and regain its function.”
Dr. Chaubey further explained, “Through our study, we demonstrated a drug-based way to achieve this in animal models, and we also identified candidate proteins in the human AD brain that may be related to the ability to reverse AD.”
Dr. Pieper emphasized that NAD+ precursors currently available over the counter have been shown in animal models to increase cellular NAD+ to dangerously high, cancer-promoting levels.
However, the approach of this study uses a pharmacological agent (P7C3-A20) that allows cells to maintain their proper NAD+ balance under conditions of overwhelming stress, without elevating NAD+ to supraphysiological levels.
“This is important when considering patient care, and clinicians should consider the possibility that therapeutic strategies aimed at restoring the energy balance of the brain may offer a path to recovery from the disease,” Dr. Pieper said.
This work also encourages new research into complementary approaches and eventual testing in patients, and the technology is being commercialized by the Cleveland-based company Glengary Brain Health, co-founded by Dr. Pieper.
“This new therapeutic approach to recovery must be translated into carefully designed human clinical trials to determine whether the efficacy observed in animal models translates to human patients,” explained Dr. Pieper.
“Additional next steps for laboratory research include identifying which aspects of the brain’s energy balance are most important for recovery, identifying and evaluating complementary approaches to reversing Alzheimer’s, and investigating whether this recovery approach is also effective in other forms of chronic age-related neurodegenerative diseases.”
Key questions answered:
A: They found that a severe decrease in NAD+ is an important driver of Alzheimer’s pathology, disrupting energy balance and damaging key brain systems.
A: Yes. In two different Alzheimer’s mouse models, restoring NAD+ balance repaired structural and functional brain damage and fully restored cognitive performance.
A: Over-the-counter NAD+ precursors can drive NAD+ to unsafe levels, but P7C3-A20 helps the brain maintain the proper balance of NAD+ under stress without causing harmful elevations.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this news about Alzheimer’s research
Author: Ansley Kelm
Source: University Hospitals Cleveland Medical Center
Contact: Ansley Kelm – University Hospitals Cleveland Medical Center
Image: Image is credited to Neuroscience News.
Original research: Open access.
“Pharmacological reversal of Alzheimer’s disease in mice reveals potential therapeutic nodes in the human brain” by Kalyani Chaubey et al. Cell Report Medicine
Abstract
Pharmacological reversal of Alzheimer’s disease in mice reveals potential therapeutic nodes in the human brain
Alzheimer’s disease (AD) is traditionally considered irreversible. Here, however, we provide proof of principle for the therapeutic reversibility of advanced AD.
In amyloid-driven 5xFAD mice with advanced disease, treatment with P7C3-A20, which restores nicotinamide adenine dinucleotide (NAD+) homeostasis, reverses tau phosphorylation, blood-brain barrier impairment, oxidative stress, DNA damage, and neuroinflammation, and enhances hippocampal neurogenesis and synaptic plasticity, resulting in complete cognitive recovery and reduced of plasma levels of the clinical biomarker of AD. p-tau217.
P7C3-A20 also reverses advanced disease in tau-driven PS19 mice and protects human brain microvascular endothelial cells from oxidative stress. In humans and mice, the severity of pathology correlates with disruption of brain NAD+ homeostasis, and the brains of non-demented individuals with Alzheimer’s neuropathology exhibit gene expression patterns suggestive of preserved NAD+ homeostasis.
Forty-six proteins aberrantly expressed in the advanced 5xFAD mouse brain and normalized by P7C3-A20 show similar alterations in the human AD brain, revealing targets with potential to optimize translation to patient care.