Summary: Researchers have designed a next-generation glutamate sensor, iGluSnFR4, capable of detecting the weakest incoming synaptic signals between neurons – signals that, until now, have been nearly impossible to record in living brain tissue. By capturing these silent inputs, scientists can finally observe how neurons weigh thousands of glutamate messages and transform them into electrical output, the central computation behind memory, learning, and emotions.
This advance opens new avenues for studying disorders marked by altered glutamate signaling and provides researchers with a powerful tool to test how potential therapies actually affect synaptic communication. The work represents an important step toward decoding the brain’s internal language and mapping how neural circuits actually work.
Key facts
New input detection: iGluSnFR4 is the first protein sensor sensitive enough to reliably record incoming glutamate signals at individual synapses in real time. Decoding Computation: Sensor reveals how neurons integrate thousands of chemical inputs to generate electrical output, illuminating core neural computations. Disease impact: Disorders such as Alzheimer’s, autism, schizophrenia, and epilepsy involve disrupted glutamate signaling; This tool provides a way to identify those disruptions directly in neural circuits.
Source: Allen Institute
Scientists have designed a protein capable of recording the incoming chemical signals of brain cells (rather than just their outgoing signals).
These silent incoming messages are the release of the neurotransmitter glutamate, which plays a critical role in how brain cells communicate with each other but has until now been extremely difficult to capture.
Why is it important
Understanding the brain’s code: Scientists can now study how neurons compute: how they take thousands of input signals and, based on them, produce an output signal that could be the basis of decision, thought or memory, decoding long-held mysteries about the brain. New avenues for disease research: Altered glutamate signaling is linked to Alzheimer’s, schizophrenia, autism, epilepsy and more. These sensors could help uncover the root causes of these conditions. Smarter drug development: Pharmaceutical companies can test how new treatments affect actual synaptic activity, accelerating the search for better therapies.
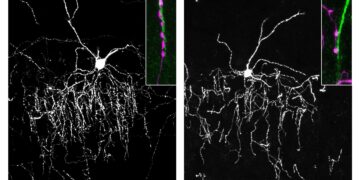
The special protein that researchers at the Allen Institute and HHMI’s Janelia Research Campus have designed is a molecular “glutamate indicator” called iGluSnFR4 (pronounced ‘glue tracker’).
It is sensitive enough to detect the weakest incoming signals between the brain’s neurons, offering a new way to decipher and interpret its complex cascade of electrical activity that underpins learning, memory and emotions.
iGluSnFR4 could help decode the brain’s hidden language and deepen our understanding of how its complex circuits work. This discovery allows researchers to observe how neurons in the brain communicate in real time.
The findings have just been published in Nature Methods and could transform the way neuroscience research is done when it comes to measuring and analyzing neural activity.
The hidden language of the brain discovered
To understand the importance of this discovery, it is useful to understand how the brain works: billions of neurons “communicate” with each other by sending pulses of electricity along their branch-like axons.
When electrical signals reach the ends of axons, they cannot jump to the next brain cell, known as a synapse. Instead, they trigger the release of chemical messengers called neurotransmitters (glutamate is the most common and critical for memory, learning, and emotions) at the synapse that causes the next brain cell to fire in sequence.
It’s like a row of falling dominoes, but much more complex: each neuron receives input from thousands of other neurons, and specific patterns and combinations of those input neurons firing are what cause the next (receiving) neuron to fire. With this new discovery, scientists can now identify the critical patterns and combinations of input neuron activity that cause subsequent neurons to fire.
Until now, detecting these incoming signals in living brain tissue was almost impossible. Older technologies were either too slow or not sensitive enough to capture action at the level of a single synapse. Now researchers can hear the entire conversation rather than fragments of it.
“It’s like reading a book with all the words jumbled up and not understanding the order of the words or how they are arranged,” said Kaspar Podgorski, Ph.D., lead author of the study and senior scientist at the Allen Institute.
“I feel like what we’re doing here is adding connections between those neurons, and in doing so, we now understand the order of words on the pages and what they mean.”
Before these protein sensors existed, researchers could only record outgoing signals from brain cells, leaving half of the communication equation (the cells’ inputs) a mystery. Incoming signals were always too weak and fast to capture, until now.
“Neuroscientists have pretty good ways of measuring the structural connections between neurons, and in separate experiments, we can measure what some of the neurons in the brain say, but we haven’t been good at combining these two types of information. It’s difficult to measure what neurons say to which other neurons,” Podgorski said.
“What we’ve invented here is a way to measure the information coming to neurons from different sources, and that’s been a critical missing part of neuroscience research.”
“The success of iGluSnFR4 arises from our close collaboration initiated at the HHMI Janelia Research Campus between the GENIE Project team and the Kaspar laboratory. That research has extended to the phenomenal in vivo characterization work performed by the Allen Institute’s Neural Dynamics group,” said Jeremy Hasseman, Ph.D., scientist at the HHMI Janelia Research Campus.
“This was a great example of collaboration between laboratories and institutes to enable new discoveries in neuroscience.”
This discovery removes a major barrier in modern neuroscience: the inability to clearly monitor and understand how brain cells receive information. With this powerful new tool available to researchers through Addgene, some of the brain’s deepest mysteries may soon be revealed.
Key questions answered:
A: They designed a protein sensor sensitive enough to record incoming glutamate signals from neurons in real time, something that was previously impossible in living brain tissue.
A: Incoming synaptic inputs determine how neurons compute and decide whether to fire, giving researchers access to the patterns underlying learning, memory, emotions, and decision-making.
A: Because disruption of glutamate signaling is implicated in disorders such as Alzheimer’s, autism, schizophrenia, and epilepsy, this sensor allows scientists to directly observe synaptic dysfunction and test how treatments alter actual neural communication.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this research news in neurotechnology and neuroscience
Author: Peter Kim
Source: Allen Institute
Contact: Peter Kim – Allen Institute
Image: Image is credited to Neuroscience News.
Original research: Open access.
“Glutamate indicators with increased sensitivity and customized deactivation rates” by Kaspar Podgorski et al. Nature Methods
Abstract
Glutamate indicators with increased sensitivity and customized deactivation rates
Understanding how neurons integrate signals from thousands of input synapses requires methods to monitor neurotransmission at many sites simultaneously.
The fluorescent protein glutamate reporter iGluSnFR allows visualization of synaptic signaling, but the sensitivity, scale and speed of such measurements are limited by existing variants.
Here we developed two highly sensitive fourth-generation iGluSnFR variants with fast activation and tailored deactivation rates: iGluSnFR4f for tracking fast dynamics and iGluSnFR4 for recording large populations of synapses.
These reporters detect glutamate with high spatial specificity and sensitivity to a single vesicle in vivo.
We used them to record natural patterns of synaptic transmission in multiple experimental contexts in mice, including two-photon imaging in cortical layers 1 to 4 and CA1 of the hippocampus, and photometry in the midbrain.
iGluSnFR4 variants expand the speed, sensitivity, and scalability of glutamate imaging, allowing direct observation of information flow through neural networks in the intact brain.