Summary: Chronic circadian disruption, such as night shift work, irregular schedules, or frequent jet lag, accelerates the development and spread of aggressive breast cancer. Researchers discovered that disrupting internal clocks not only weakens immune defenses but also remodels healthy breast tissue, creating conditions that tumors exploit.
A key immunosuppressive receptor, LILRB4, emerged as a crucial “off switch” that cancer exploits when rhythms are disrupted. Blocking this molecule reduced tumor growth and metastasis, even when the circadian system remained altered. These findings highlight sleep timing and circadian health as overlooked factors in cancer progression and point toward new therapeutic strategies.
Key facts:
Clock disruption accelerates cancer: models with altered circadian rhythms developed tumors earlier and showed more aggressive spread. Immune suppression pathway: circadian disruption elevated the activity of the immunosuppressive receptor LILRB4, creating a favorable environment for tumors. New therapeutic angle: targeting LILRB4 reduced metastasis even under altered circadian conditions.
Source: Texas A&M
Working the night shift, frequently flying across time zones, or maintaining an irregular sleep schedule does more than leave us exhausted; may increase the risk of aggressive breast cancer. Exactly how and why this happens remains a mystery until now.
A new study from the College of Arts and Sciences at Texas A&M University, led by Dr. Tapasree Roy Sarkar, sheds light on this elusive link, finding that circadian disruptions change the structure of the mammary glands and weaken the immune system’s defenses, while pointing toward a new way to counteract these effects.
“Cancer keeps time,” Sarkar said. “If your internal clock is disrupted, cancer takes advantage, but now we have found a new way to fight back.”
When the clock is off, the danger is on
Circadian rhythms (our internal 24-hour clock) do much more than regulate sleep. They help coordinate hormone release, tissue repair, and immune system surveillance.
When they are altered, the body’s natural defenses begin to weaken.
“The circadian rhythm organizes how our tissues function and how our immune system recognizes danger,” Sarkar said. “When that rhythm is disturbed, the consequences can be seriously dangerous.”
To investigate these effects, the researchers used two groups of genetically engineered models that develop aggressive breast cancer. One group lived on a normal day-night schedule, while the other lived on a disrupted light cycle that disrupted their internal clocks.
The findings, published in the Nature group’s journal Oncogene, were surprising.
Typical models develop cancer around the 22-week marker. However, the circadian-disrupted group showed signs of cancer much earlier, at almost 18 weeks.
Tumors in circadian-disrupted models were also much more aggressive and much more likely to spread to the lungs, a key indicator of poor outcomes in breast cancer patients.
At the same time, disrupting the models’ internal clock suppressed immune defenses, creating a more hospitable environment for cancer growth.
“It wasn’t just that the tumors were growing faster,” Sarkar said. “The immune system was actively restricted, creating more favorable conditions for cancer cells to survive and spread.”
But the effects were not limited only to the tumors themselves. The researchers also found that long-term circadian disruption changed the composition of healthy breast tissue, making it more vulnerable to cancer.
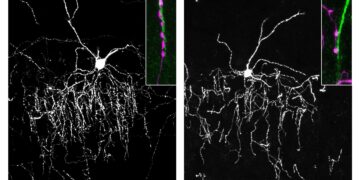
“We observed clear changes in the morphology of the mammary glands, the milk-producing tissue of the breast,” Sarkar said.
Immune ‘off switch’ revealed
To understand how circadian disruptions affected healthy breast tissue and suppressed immune defenses, the researchers took a closer look inside the tumors.
One molecule stood out: leukocyte immunoglobulin-like receptor B4 (LILRB4), a receptor already known to suppress immune responses in several cancers.
Under normal conditions, LILRB4 helps prevent excessive inflammation and protects healthy tissue.
In the case of cancer, however, it can accelerate and become dangerous. Think of LILRB4 as the immune system’s “off switch.”
“LILRB4 acts as an immune checkpoint,” Sarkar said. “When we targeted LILRB4, the tumor microenvironment became less immunosuppressive and even under altered circadian conditions, we observed reduced cancer spread.”
Deactivating this immune checkpoint helped restore the immune system’s ability to defend itself, suggesting a new therapeutic angle for the treatment of aggressive breast cancers related to circadian disruptions.
“When we started to intervene and regulate the activity of LILRB4, we saw significantly less cancer metastasis and tumor growth,” Sarkar said.
A new context for a known goal: personalized cancer treatment
By experimentally linking circadian alterations to breast cancer progression, the study opens new doors to targeted therapies for patients whose lifestyles or occupations place them at chronic circadian risk.
“This study shows what can happen when our internal clock is repeatedly disrupted and how we might begin to repair the damage,” Sarkar said.
It also provides some of the strongest evidence that circadian disruption not only correlates with cancer risk, but may also actively drive cancer progression.
“The study reframes sleep and schedule as powerful players in cancer progression and treatment,” Sarkar said.
And in a world that actively operates 24 hours a day, the implications extend far beyond the laboratory. An estimated 12 to 35 percent of Americans work irregular hours, including night and rotating shifts.
“A significant portion of the population works at night or on rotating shifts,” Sarkar said. “This makes understanding the impact of circadian disruptions on cancer risk incredibly important.”
The research team’s next big project is to investigate how the effects of chronic circadian disruptions could be reversed in humans, with the goal of improving health outcomes for night shift workers and other people with irregular sleep schedules, such as flight attendants and frequent travelers.
“Our next goal is to better understand how we can reverse the effects of circadian disruption and help improve human health with real-world impact,” Sarkar said.
Cancer may keep time, but with monumental discoveries like these, scientists are learning to take control of the clock.
Key questions answered:
A: Disruption of sleep-wake rhythms weakens immune defenses, alters the structure of breast tissue and creates conditions that allow tumors to grow earlier and spread more aggressively.
A: LILRB4 functions as an immune “off switch” that becomes overactive during circadian disruption, suppressing immune surveillance and allowing tumors to thrive. Blocking it reduces metastasis.
A: Chronic rhythm misalignment puts pressure on the immune and tissue repair systems, making the body more vulnerable to tumor development and accelerating cancer progression over time.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this circadian rhythm and the news about cancer research
Author: Zaid Elayyan
Source: Texas A&M
Contact: Zaid Elayyan – Texas A&M
Image: Image is credited to Neuroscience News.
Original research: Open access.
“LILRB4 regulates circadian disruption-induced mammary tumorigenesis through a non-canonical WNT signaling pathway” by Tapasree Roy Sarkar et al. oncogene
Abstract
LILRB4 regulates circadian disruption-induced mammary tumorigenesis through the non-canonical WNT signaling pathway
Epidemiological studies have shown that circadian rhythm disruption (CRD) is associated with breast cancer risk. However, the role of CRD in mammary gland morphology and aggressive basal mammary tumorigenesis and the molecular mechanism underlying CRD-induced carcinogenesis remain unknown.
To investigate the effect of CRD on aggressive tumorigenesis, a genetically engineered mouse model of aggressive breast cancer was used. The impact of CRD on the tumor microenvironment was investigated using tumors from LD12:12 and CRD mice using scRNA-seq, flow cytometry, multiplexed immunostaining, and real-time PCR. The effect of LILRB4 immunotherapy on CRD-induced tumorigenesis was also investigated.
Here we investigated and identified the impact of CRD on basal tumorigenesis and mammary gland morphology.
We found that chronic CKD altered mammary gland morphology, increased lung metastasis, and induced an immunosuppressive tumor microenvironment by enhancing LILRB4 expression.
Furthermore, immunotherapy targeting LILRB4 reduced the CRD-induced immunosuppressive microenvironment and lung metastasis. Finally, we demonstrate that LILRB4 regulates CRD-induced mammary tumorigenesis through a non-canonical WNT signaling pathway.
These findings identify and implicate LILRB4 as a link between CKD and aggressive breast tumorigenesis and establish the potential role of immunotherapy targeting LILRB4a as an inhibitor of CKD-induced lung metastasis.