Summary: New research shows that childhood environments shape lifelong memory through a single molecular switch that controls genetic activity related to learning. In animal models, enriched early experiences activated this switch, strengthening neural circuits involved in memory and cognition, while deprived environments suppressed it.
Blocking this molecular regulator completely erased the cognitive benefits of stimulation. The results reveal how life experiences are biologically integrated in the brain and may guide future therapies for cognitive and neurodevelopmental disorders.
Key facts
Molecular switch identified: A single transcription factor translates early stimulation into lasting changes in memory. The environment shapes the brain: enriched conditions strengthened learning circuits, while impoverished conditions weakened them. Therapeutic potential: The mechanism could aim to mimic the cognitive benefits of enriched environments.
Source: UMH
A team from the Institute of Neurosciences (IN), a joint research center of the Higher Council for Scientific Research (CSIC) and the Miguel Hernández University of Elche (UMH), led by researcher Ángel Barco, has identified a molecular mechanism that helps explain why growing up in a stimulating environment improves memory. On the contrary, a lack of stimulation can harm it.
The study, carried out in mice and published in Nature Communications, demonstrates that the environment during childhood and adolescence has a lasting impact on the brain by activating or repressing a single transcription factor, AP-1, which regulates the expression of genes involved in neuronal plasticity and learning.
This finding identifies a molecular mediator that can translate life experiences into persistent changes in cognitive function.
To carry out the research, the team from the IN’s Transcriptional and Epigenetic Mechanisms of Neuronal Plasticity laboratory raised young mice in three different conditions: an environment enriched with toys, exercise wheels and social interaction; a standard environment; and an impoverished environment characterized by isolation and lack of stimulation.
After several weeks, animals raised in enriched environments showed superior performance on learning and memory tasks, while those raised in impoverished environments scored lower on cognitive tests.
Using advanced genomic and epigenetic techniques to analyze the brain, the researchers observed that early life experiences produce a lasting modulation of AP-1 activity: its activation stimulates gene networks that strengthen neuronal connections, while reduced activity weakens those same processes.
To functionally validate this finding, the team experimentally blocked the Fos gene, one of the essential subunits of the AP-1 complex. Under these conditions, the mice did not benefit from the enriched environment. They showed no cognitive improvement, demonstrating that AP-1 not only correlates with environmentally induced changes in the brain, but is also necessary for them to occur.
“We have known for decades that the early life environment influences learning ability, but we lacked a clear mechanism to explain how this happens. Now we have identified a molecular switch that translates those early experiences into lasting changes in the brain,” explains Barco.
“What is striking is that a single transcription factor acts as a point of convergence for experiences as diverse as sensory stimulation, exercise or social interaction. It is a key piece to understanding how the environment shapes memory,” says the leader of the study.
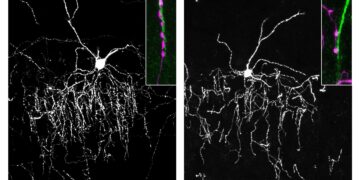
The study also reveals that environmental impact varies between neuronal populations. By analyzing specific types of neurons, the scientists found that AP-1 responds differently in CA1 pyramidal neurons and granule cells of the dentate gyrus, two key populations involved in spatial learning and memory formation.
According to Marta Alaiz-Noya, co-first author of the study along with Federico Miozzo and Miguel Fuentes Ramos, “the strong activation of AP-1 in enriched environments triggers genetic programs that allow the brain to enter ‘learning mode’, reinforcing neuronal connections during particularly sensitive stages of development.”
“Together, these findings reinforce the idea that environmental stimulation and social interaction during childhood and adolescence not only enrich the life experience, but also leave a tangible biological imprint on the brain. Furthermore, they open the door to future therapeutic strategies that mimic the effects of enriched environments in neurodevelopmental disorders or in conditions involving cognitive impairment,” adds Federico Miozzo.
The paper also involved researchers from the Faculty of Mathematics, Computer Science and Mechanics at the University of Warsaw, Poland, who contributed to the bioinformatic analysis of DNA methylation data in the three settings.
Financing: The work has been possible thanks to the financing of the “la Caixa” Foundation, the State Research Agency – Ministry of Science, Innovation and Universities, the Carlos III Health Institute, the European Regional Development Fund (ERDF) of the European Union and the Generalitat Valenciana.
Key questions answered:
A: Early sensory, social and physical stimulation activates a transcription factor that permanently strengthens neuronal plasticity.
A: Reduced activation of this molecular switch weakens genetic networks related to learning and impairs memory.
A: The findings suggest that future treatments could mimic enriched environments at the molecular level.
Editorial notes:
This article was edited by a Neuroscience News editor. Magazine article reviewed in its entirety. Additional context added by our staff.
About this news about research in memory, genetics and neurodevelopment
Author: Ángeles Gallar
Source: UMH
Contact: Ángeles Gallar – UMH
Image: Image is credited to Neuroscience News.
Original research: Open access.
“Modulation of cognition and neuronal type-specific AP-1 signaling in early rearing conditions” by Ángel Barco et al. Nature Communications
Abstract
Neuronal-specific modulation of cognition and AP-1 signaling under early-life rearing conditions
Environmental conditions profoundly influence cognitive development, especially during the first years of life.
Transcriptional and epigenetic mechanisms may serve as molecular substrates for the long-lasting effects of environmental enrichment (EE) and impoverishment (IE) on cognitive abilities and hippocampal function. However, the specific genetic programs driving these changes remain largely unknown.
In this study with female mice, EE and IE produced opposite effects on cognitive performance.
By combining hippocampal microdissection and genetic labeling of neuronal nuclei with genome-wide analyzes of gene expression, chromatin accessibility, histone acetylation, and DNA methylation, we uncovered profound differences in the transcriptional and epigenetic profiles of CA1 pyramidal neurons and dentate gyrus (DG) granule neurons.
These analyzes revealed cell type-specific genomic changes induced by EE and IE, highlighting distinct patterns of neuroadaptation within each population. This multi-omics screen identified the activity-regulated transcription factor AP-1 as a crucial mediator of neuroadaptation to conditions during early life in both cell types, albeit through distinct downstream mechanisms.
Conditional deletion of Fos, a core subunit of AP-1, in excitatory neurons hampered EE-induced cognitive improvement, further underscoring the critical role of this transcription factor in neuroadaptation.