_6e98296023b34dfabc133638c1ef5d32-620x480.jpg)
A human phase 1 clinical trial to treat chronic spinal cord lesion, the first of its kind in the world, has begun to prove the effectiveness and safety of a new revolutionary treatment using nasal cells.
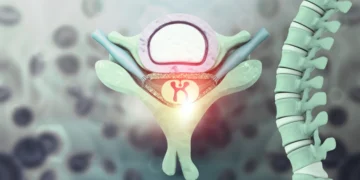
The essay of the University of Griffith has been in three decades in creation and implies taking olfactory cells, which are specialized cells involved in our sense of smell, from the nose, since they have numerous therapeutic properties to repair and regenerate the nerves.
Principal researcher, Professor James St. John, head of the Griffith stem cell neurobiology and research Center, the principal researcher and principal researcher of the Institute of Biomedicine and Glycomics, is leading to the legacy of the late Professor Emeritus Alan Mackay-Sim AM.
“Once the cells have been removed from the patient’s nose, they are used to create an innovative nervous bridge that is approximately the size of a very small worm,” said Professor St. John.
“The nervous bridge is implanted in the column on the site of the lesion, offering what we believe is the best hope to treat the spinal cord injury.
“To help stimulate regeneration, patients will undergo intensive rehabilitation for three months before transplantation and then for eight months after transplantation.
“While primary evaluations are to ensure that therapy is safe, we will also measure numerous aspects to assess whether there are changes in functional results that are important for people who live with spinal cord injury.
“The ability to recover a sense of function, whether to recover the independent function of your bladder or intestine, recover movement in your fingers or the ability to stop and embrace a loved one can again improve the quality of life.
“Recovering some form of independence can open the world to people who live with an injury in the chronic column acquired.”
The essay, which will be held at the Hospital of the University of Gold Coast, is a blind and random control study with a preclinical investigation that shows that olfactory nerve bridges are effective in repairing the spinal cord injury in animal models.
The CEO of the Clem Jones Foundation, Peter Johnstone, said that the last milestone illustrates how long -term philanthropic support could promote innovative investigation with the potential to change lives for the better.
The Clem Jones Foundation has supported this world leader from the first day together with other philanthropic groups and individuals, which meant that it also attracted the financing commitments of the state and federal government.
All financing partners recognize that the results of medical research never occur overnight, but trust long -term financing, as well as in the long -term application of knowledge, skills and hard work of the talented team of researchers from the University of Griffith. “
Peter Johnstone, CEO of the Clem Jones Foundation
The founder of the Perry Cross Spinal Research Foundation, Perry Cross AM, who became a ventilated tetraple at 19 years of a rugby accident, has dedicated his life to advocate by a cure.
“This clinical trial represents a long -awaited advance that speaks of the enduring force of those affected by the spinal cord injury and the extraordinary belief of those who support us,” Cross said.
“For too long, people who live with paralysis have been told that recovery is beyond the horizon of the possibility.
“Today we challenge that notion with evidence, ambition and, above all, hope.
“It is proof that philanthropy, when guided with a purpose and vision, can accelerate real change. Each contribution has imported, and each support gesture has brought us closer to this point.
“For someone like me, who knows very well the permanence of the spinal cord injury, this judgment offers not only the possibility of an improved function, but a renewed sense of independence and dignity; qualities that define human experience.”
Professor St. John said: “Having a cell transplant therapy that progresses to clinical trial after only eight years is a testimony of the benefits of the strategic translation research program that the team has used.
“Being able to develop the therapy in Queensland is thanks to the incredible support of our financing partners, in particular the motor accident insurance commission such as the main financier, the Clem Jones Foundation, the Espinal Perry Cross’s research foundation, the National Council of Health and Medical Research, future medical research fund and the dedicated community of spinal injuries that has been inspiration and the driving force behind the development of the therapy.”
The trial is funded by the future fund of medical research, the Perry Cross Spinal Research Foundation, the Clem Jones Foundation, the Queensland government, Nicola and Andrew Forrest, Brazil Family Foundation, Terry and Rhonda White and the University of Griffith.
(Tagstotranslate) Clinical trial