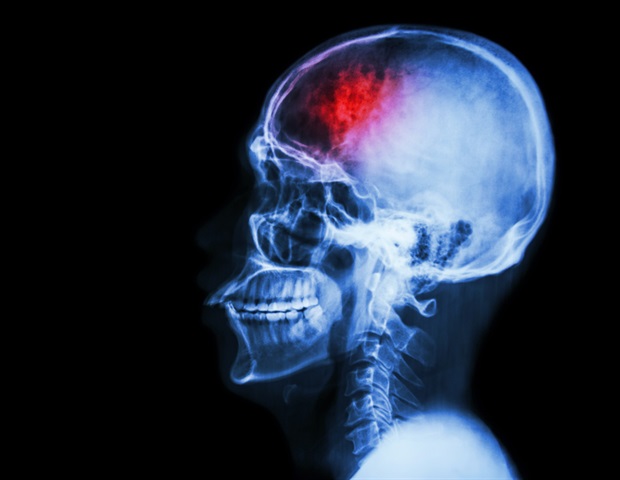
Two widely favorite drugs from Alzheimer were shown to enable patients to stay in their homes, living independently, for longer periods of time.
However, these medications, though effective, are not without their risks and side effects.
This is according to a recent study published last week in the Alzheimer’s Association Journal.
Red meat could risk dementia, researchers claim, yet some doctors have any questions
Researchers at Washington University.
Two widely favorite drugs from Alzheimer were shown to enable patients to stay in their homes, living independently, for longer periods of time. (istock)
Both FDA-approved drugs are designed to remove amyloid beta plaques from the brain of people with an early stage Alzheimer’s disease, possibly slowing the rate of cognitive decline.
Patients who took Lecaemab extended their time at home for another 10 months, while Donanemab enabled them to live independently for eight more months, according to Washu press release.
Memory loss is not always alzheimer: experts warn of common but little known dementia
On average, a patient with mild symptoms could expect to live independently for another 29 months without treatment, 39 months with lecaemab and 37 months with Donanemab, according to the edition.
These results were based on patients who began treatment with “very mild symptoms.”
“If you think about the cost and consequences of not being able to live independently, this can be significant for many older adults.”
“Using the data from the clinical trials, we calculated that for an average patient with very mild symptoms due to Alzheimer , a professor of psychiatry at Washu Medicine, told Fox News Digital.
“If you think about the cost and consequences of not being able to live independently, this can be significant for many older adults.”

On average, a patient with mild symptoms could expect to live independently for another 29 months without treatment, 39 months with lecaemab and 37 months with Donanemab. (istock)
This study gives patients and their families a way of translating clinical test findings into significant results, according to Hartz.
“For example, if a patient is considering taking a lecaemab or Donanemab to deal with his advertisement, part of the discussion with his doctor may be how long they would expect that the drug will extend its independence, so to be able to live independently and be able to independently take care of their own bodies .
3 characters that your aging loved one may be ready for a helped life
The purpose of the study is not to plead for or against these drugs, Hartz noted in a press release. “The goal … is to put the effect of these medicines in context in ways that can help people make the decisions best for themselves and their family members.”
Christopher Weber, Doctor, senior director of global scientific initiatives at the Alzheimer’s Association, was not involved in the study, but shared his input with Fox News Digital.

Anyone who considers these treatments must have a “deep conversation” with a doctor about the possible benefits and weigh them against the “significant risks”, according to a doctor. (istock)
“This study suggests that these drugs have a clinically significant impact on Alzheimer’s early patients and their families providing more time in the early stages of the disease, when patients have more functional independence,” he said.
The findings of the study emphasize the importance of starting treatment early to maximize the possible benefits, according to Weber, as starting at a more advanced point resulted in fewer months of independence.
Click here to get the Fox News app
“The advances we see in Alzheimer’s treatments are based on decades of research, and emphasize the importance of ongoing innovation and investment in this field to better understand the underlying biology and trajectory of the disease, detect it earlier, And effectively treat and prevent it, “he added.
Possible risks and restrictions
The biggest limitation of the study, according to Hartz, is that the participants were very dedicated to Alzheimer’s research and were typically highly educated.
“We don’t know how well our findings translate to the rest of the population,” she acknowledged.
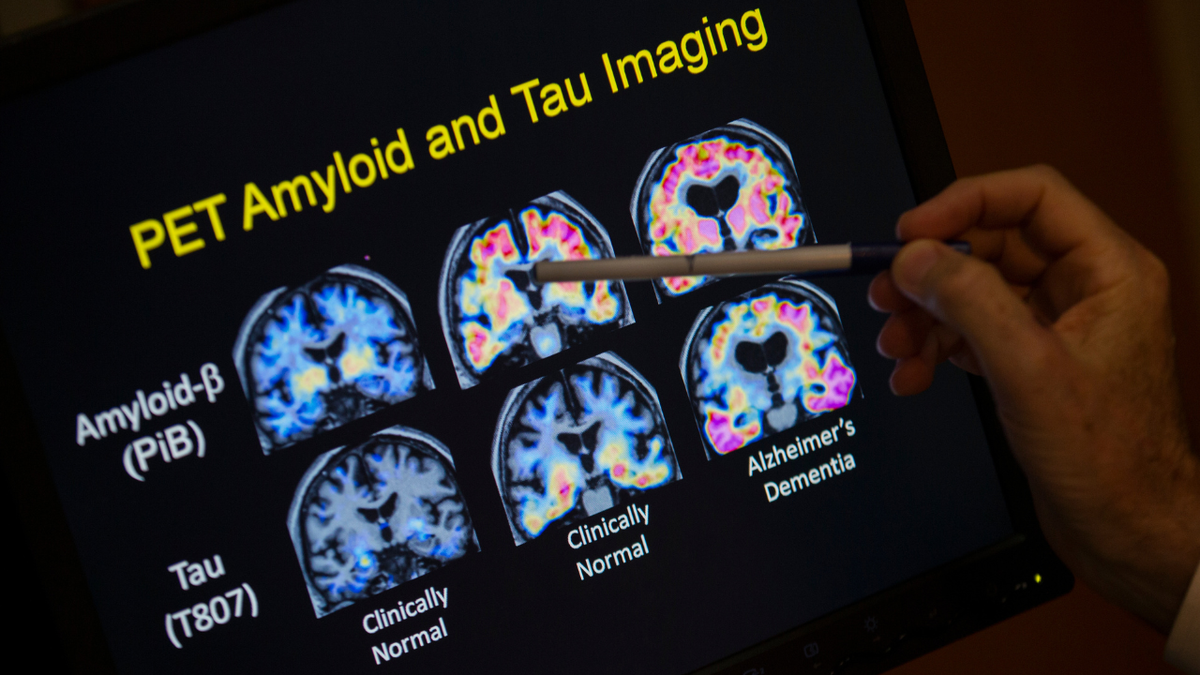
Both FDA-approved drugs are designed to remove amyloid beta plaques from the brain of people with an early stage Alzheimer’s disease, possibly slowing the rate of cognitive decline. (AP Photo/Evan Vucci, File)
Dr. Chris Vercammen, board member-certified internal medical doctor, who specializes in geriatric and palliative care, emphasized that while the two drugs can extend periods of independence for people with Alzheimer, “LeCanemab and Donanemab do not stop or reverse Alzheimer’s disease. ”
“It is also important to understand that these new drugs are only useful for individuals in the early stages of the disease,” the doctor, who is also a medical director at Remo Health in California, told Fox News Digital. (Vercammen was not involved in the new study.)
Click here to register for our health information
Anyone who considers these treatments must have a “deep conversation” with a doctor about the possible benefits and weighing them against the “significant risks” involved, according to Vercammen.
“These risks include the possibility of major side effects, such as brain swelling and bleeding, as well as the considerable financial costs associated with treatment, even if you have insurance coverage,” he warned.
“These new drugs are only useful for individuals in the early stages of the disease.”
For people who may benefit from these treatments, Hartz recommends that they talk to their doctors and review the possible risks and benefits.
For more health articles, visit www.foxnews.com/health
“This information could help contextualize how the drugs can benefit them about independence,” she added.
Fox News Digital reached the manufacturers of Leqembi and Kisunla to apply for a comment.