BOCA RATON, Fla., August 27, 2024–(BUSINESS WIRE)–SurGenTec, an progressive medical system firm specializing in orthopedic and spine expertise, is thrilled to announce that it has acquired 510(ok) clearance from the U.S. Food and Drug Administration (FDA) for its proprietary B-MAN Bone Marrow Aspirate Equipment.
The B-MAN Equipment introduces a cutting-edge strategy to bone marrow aspiration, using a centrifuge-free technique for the gathering of high-quality aspirate with minimal contaminants. Its built-in diamond-tip trocar works concurrently with CELLect filtration expertise, which successfully reduces bone spicules and different impurities, leading to refined aspirate with enhanced cell viability.
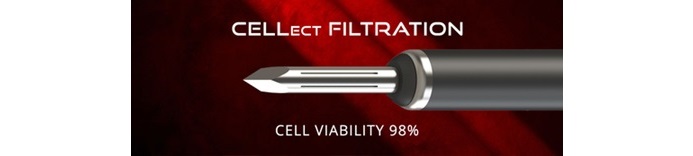
“We’re excited so as to add this distinctive system to our product portfolio,” stated Travis Greenhalgh, CEO of SurGenTec. “Our goal was to create a minimally invasive answer that simplifies the aspiration course of whereas optimizing cell high quality. The B-MAN Equipment preserves native cell construction and facilitates the extraction of more healthy cells by decreasing the manipulation that takes place, offering physicians with a strong autologous possibility for affected person therapy.”
Conventional bone marrow aspiration strategies usually contain a number of steps and the usage of centrifuges, which might negatively affect cell well being. The B-MAN Equipment streamlines this course of by selling ease of use and effectivity whereas accessing bone marrow.
By maximizing marrow contact with a floor space as much as 3 times better than standard strategies, the B-MAN Equipment enhances the retrieval of progenitor cells whereas decreasing contamination with peripheral blood and bone spicules. This progressive design positions the B-MAN Equipment as a robust device for healthcare suppliers searching for to deal with their sufferers.
Bone marrow aspirate performs a important function in numerous surgical purposes, together with orthopedic procedures, spinal interventions, and regenerative drugs. Using a affected person’s personal cells considerably lowers the chance of illness transmission in comparison with merchandise derived from allografts or donors. Because the regenerative drugs market sector continues to evolve and develop, the B-MAN Equipment affords a invaluable possibility for physicians aiming to boost affected person care.
SurGenTec is a privately owned medical system firm based mostly in Boca Raton, Florida, specializing in distinctive applied sciences for orthopedic and neurosurgical spine remedies. They’re dedicated to offering minimally invasive therapy choices that prioritize affected person security. SurGenTec has secured quite a few nationwide GPO agreements all through the US and has entered the worldwide market. SurGenTec will proceed to develop its portfolio via its in-house engineering incubator, with extra product releases all through 2024 and into 2025.
For extra details about the B-MAN Bone Marrow Aspirate Equipment and SurGenTec’s progressive options, please go to www.surgentec.com.
Contacts
customerservice@surgentec.com



















Discussion about this post