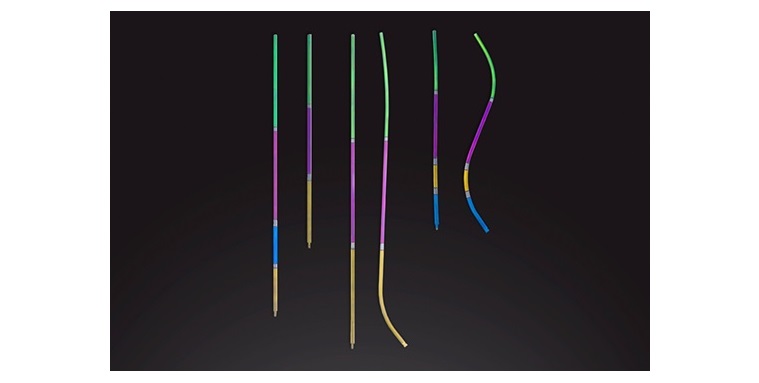
SANTA CLARA, Calif., Oct. 10, 2024 (GLOBE NEWSWIRE) — SI-BONE, Inc. (Nasdaq: SIBN), a Silicon Valley-based medical machine firm devoted to offering surgical options for sacropelvic issues, in the present day introduced first-in-patient procedures with the FDA-designated breakthrough machine, iFuse TORQ TNT™ Implant System (TNT). Designed to deal with the anatomic and biomechanical challenges of pelvic fragility fractures, significantly in sufferers with poor bone high quality, TNT provides a big development over conventional cannulated screws.
TNT, which obtained 510(okay) clearance in August 2024 and was awarded Breakthrough Machine Designation by the FDA, is the primary 3D-printed transiliac-transsacral screw cleared for market use within the U.S. It incorporates a pelvis-specific design to enhance preliminary fixation and scale back the chance of screw backout. Among the many first surgeons to carry out procedures with TNT have been Edward Westrick, MD, at Allegheny Normal Hospital in Pittsburgh, PA, Reza Firoozabadi, MD, at Harborview Medical Heart in Seattle, WA, J.D. Black, MD, at Kadlec Regional Medical Heart in Richland, WA, and Brian Cunningham, MD, at Methodist Hospital – HealthPartners in St. Louis Park, MN.
“TNT went past my expectations,” mentioned Dr. Black. “The streamlined instrumentation and implant design not solely supplied wonderful fixation but additionally allowed for fast, exact implantation. This effectivity is important when treating sufferers with fragile bones, because it reduces working time, minimizes dangers, and results in sooner restoration.”
“TNT’s 3D-printed porous floor facilitates osseointegration, which I consider will result in higher long-term outcomes for my older, osteoporotic sufferers,” mentioned Dr. Cunningham, Vice Chair and Director of Orthopedics at Methodist Hospital – HealthPartners.
“We’re thrilled with the profitable completion of those preliminary procedures utilizing our iFuse TORQ TNT system,” mentioned Laura Francis, Chief Government Officer of SI-BONE. “This breakthrough know-how marks a big step ahead in addressing the unmet medical wants of complicated pelvic fragility fractures. By offering an answer that improves each surgical effectivity and affected person restoration, we’re additional increasing our management within the sacropelvic area.”
About SI-BONE, Inc.
SI-BONE (NASDAQ: SIBN) is a world chief in know-how for the surgical therapy of sacropelvic issues. Since pioneering minimally invasive surgical procedure of the SI joint in 2009, SI-BONE has supported over 3,900 surgeons in performing greater than 100,000 sacropelvic procedures. A novel physique of medical proof helps the usage of SI-BONE’s applied sciences, together with two randomized managed trials and over 135 peer-reviewed publications. SI-BONE has leveraged its management in minimally invasive SI joint fusion to commercialize novel options for adjoining markets, together with grownup deformity, spinopelvic fixation, and pelvic trauma.
For added info on the corporate or its merchandise, together with dangers and advantages, please go to www.si-bone.com.
iFuse TORQ, iFuse Bedrock Granite, and SI-BONE are registered logos; iFuse TORQ TNT is a trademark of SI-BONE, Inc. ©2024 SI-BONE, Inc. All Rights Reserved. Edward Westrick, MD, Reza Firoozabadi, MD, and J.D. Black, MD, are paid consultants for SI-BONE, Inc. Brian Cunningham, MD, isn’t a paid guide for SI-BONE, Inc.
Investor Contact: Saqib Iqbal buyers@si-bone.com



















Discussion about this post