The U.S. Food and Drug Administration (FDA) has accredited a brand new medicine for folks with Alzheimer’s illness.
Eli Lilly’s Kisunla (donanemab) is a once-monthly injection meant for adults with early symptomatic Alzheimer’s illness, in response to a press launch from the corporate.
Eligible sufferers embrace these with delicate cognitive impairment (MCI) and people who have delicate dementia with confirmed amyloid pathology.
EXPERIMENTAL ALZHEIMER’S DRUG GETS FDA ADVISORY PANEL’S THUMBS-UP: ‘PROGRESS IS HAPPENING’
That is the primary medicine to focus on amyloid plaques — the proteins that construct up within the brains of Alzheimer’s sufferers, typically impairing reminiscence and cognitive operate — with proof to help stopping remedy when amyloid plaques are eliminated, the discharge said.
“As soon as-monthly Kisunla is the primary and solely amyloid plaque-targeting remedy with proof to help stopping remedy when amyloid plaques are eliminated,” an Eli Lilly and Firm spokesperson informed Fox Information Digital in an e mail.
The U.S. Food and Drug Administration (FDA) has accredited a brand new medicine for folks with Alzheimer’s illness. (iStock)
“The potential to finish Kisunla remedy after a limited-duration course of remedy primarily based on amyloid pathology, together with 30-minute infusions as soon as per thirty days, may imply decrease affected person out-of-pocket remedy prices and decreased infusion burden in comparison with different amyloid-targeting therapies.”
Kisunla demonstrated “very significant outcomes” for folks with early symptomatic Alzheimer’s illness, famous Anne White, govt vice chairman and president of Lilly Neuroscience, Eli Lilly and Firm, in a press launch.
CAN WE REVERSE ALZHEIMER’S DISEASE? EXPERTS SUGGEST ‘NEW PARADIGM’ FOR COMBATING DEMENTIA
“We all know these medicines have the best potential profit when individuals are handled earlier of their illness, and we’re working onerous in partnership with others to enhance detection and analysis,” she added.
The approval follows an advisory panel’s advice of the drug on the FDA’s Peripheral and Central Nervous System Advisory Committee listening to final month.
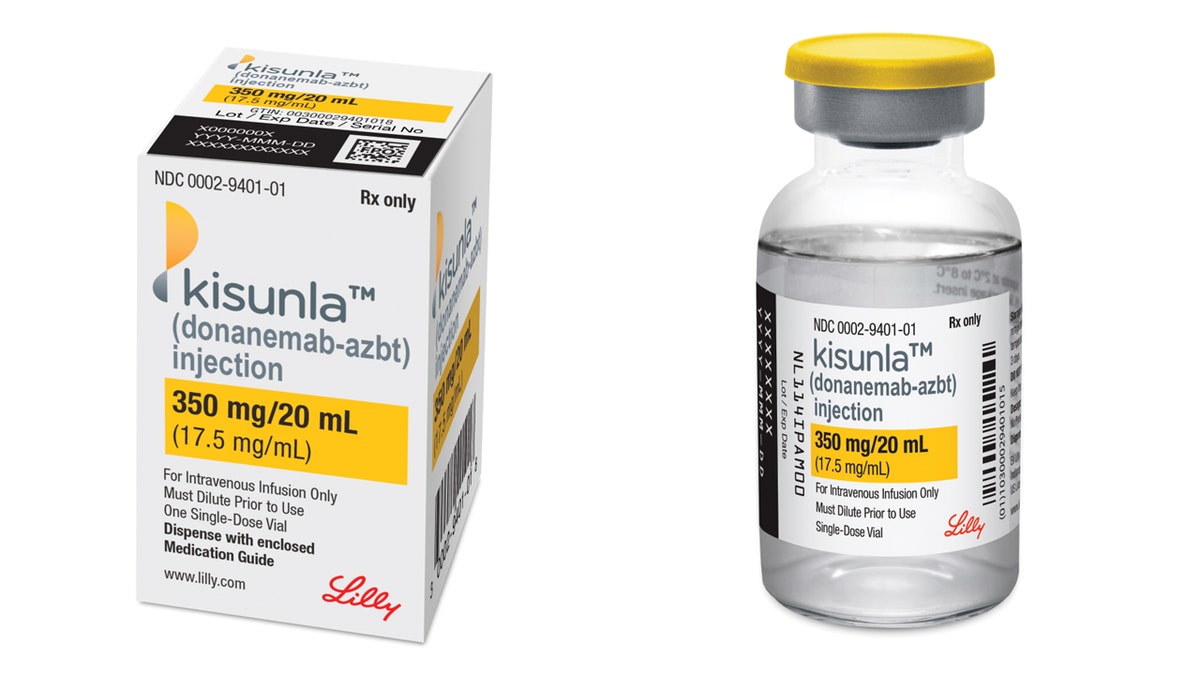
Eli Lilly’s Kisunla (donanemab) is a once-monthly injection meant for adults with early symptomatic Alzheimer’s illness. (Eli Lilly & Firm)
At that listening to, Eli Lilly officers offered scientific trial outcomes that confirmed the drug slowed cognitive and purposeful decline for folks with delicate cognitive impairment attributable to early levels of Alzheimer’s.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
In section 3 trials revealed in Might 2023, donanemab was proven to “considerably gradual cognitive and purposeful decline in folks with early symptomatic Alzheimer’s illness,” in response to a press launch on Eli Lilly’s web site.
That examine was revealed by the Journal of the American Medical Affiliation.

In section 3 trials revealed in Might 2023, donanemab was proven to “considerably gradual cognitive and purposeful decline in folks with early symptomatic Alzheimer’s illness,” in response to a press launch on Eli Lilly’s web site. (iStock)
Charles Bernick, M.D., employees neurologist at Cleveland Clinic Lou Ruvo Heart for Mind Health in Las Vegas, Nevada, famous that donanemab is in a category of medication termed “monoclonal antibodies.”
“Antibodies are produced by the immune system and permit removing of particular toxins from the physique,” Bernick informed Fox Information Digital.
“Donanemab is an antibody that particularly targets the amyloid plaques that happen in Alzheimer’s illness and causes their removing. Research present that removing of the plaques by donanemab can gradual development of the illness.”
“The group that responded one of the best have been these with the mildest signs.”
Bernick reiterated that donanemab is meant to be prescribed in people with delicate signs of Alzheimer’s illness, both those that are nonetheless functioning independently or solely want slight help.
“In truth, the group that responded one of the best have been these with the mildest signs,” he added. “It isn’t indicated for folks with extra superior signs of Alzheimer’s illness.”
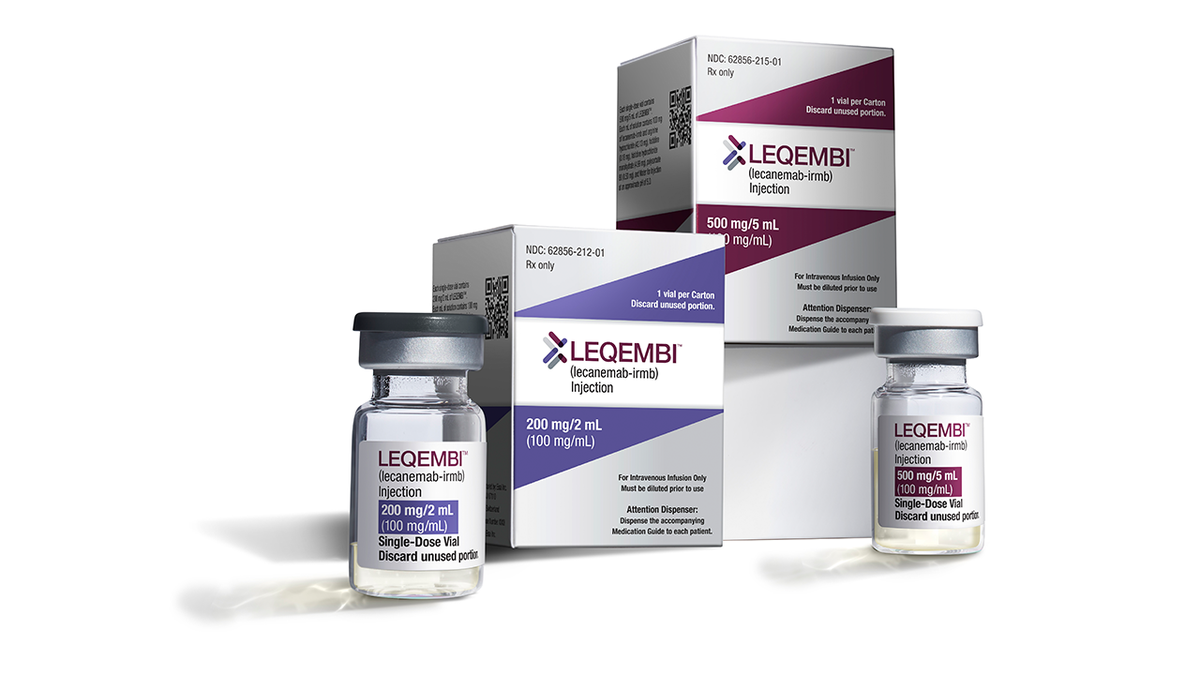
Leqembi, the primary drug to indicate that it slows Alzheimer’s, was accredited by the U.S. Food and Drug Administration in early January 2023. (AP Photographs)
Probably the most critical potential aspect impact of Kisunla is amyloid-related imaging abnormalities (ARIA), which may result in short-term mind swelling or bleeding.
Though this impact “normally resolves over time,” it may be life-threatening, the corporate stated.
Some sufferers may additionally expertise complications or probably critical allergic reactions throughout or shortly after the drug’s infusion.
CLICK HERE TO GET THE FOX NEWS APP
Dr. Marc Siegel, scientific professor of medication at NYU Langone Medical Heart and a Fox Information medical contributor, who was not concerned within the drug trials, famous that donanemab is similar to Leqembi, the present drug in the marketplace that blocks amyloid formation.
Denonemab is “considerably simpler,” Siegel famous, because it slows the development of Alzheimer’s by about 35% versus 27% for Leqembi.
“It might be higher at eradicating plaques,” he stated.
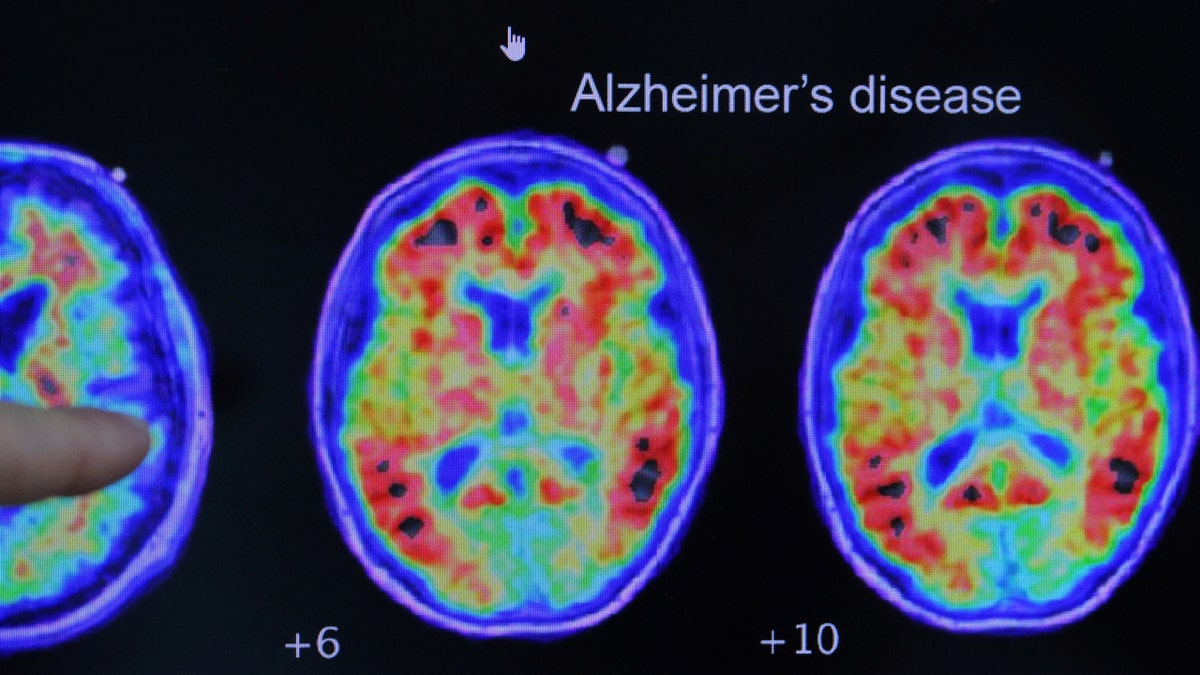
A physician factors out proof of Alzheimer’s illness on PET scans on the Heart for Alzheimer Analysis and Remedy at Brigham And Ladies’s Hospital in Boston, Massachusetts. (REUTERS/Brian Snyder/File Picture)
Each donanemab and lecanemab are efficient at eradicating amyloid plaques from the mind, Bernick informed Fox Information Digital.
“They differ primarily in that donanemab is given by infusion as soon as a month, whereas lecanemab is twice a month,” he stated.
For extra Health articles, go to www.foxnews/well being
“As well as, the beneficial remedy course of lecanemab is eighteen months, however donanemab is given solely as much as the purpose when the amyloid plaque is eliminated after which is stopped, even when this happens at six or 12 months.”
Provided that it is just given as soon as a month and is stopped as soon as plaque is eliminated, each the monetary price and burden of infusions are possible much less with donanemab, in response to Bernick.
















Discussion about this post