Scientists on the Eli and Edythe Broad Middle of Regenerative Drugs and Stem Cell Analysis at UCLA have uncovered an sudden function for the molecule netrin1 in organizing the growing spinal cord.
The researchers found that netrin1, which is thought primarily as a steerage cue that directs rising nerve fibers, additionally limits bone morphogenetic protein, or BMP, signaling to particular areas of the spinal cord. This boundary-setting perform is vital as a result of this signaling exercise should be exactly confined to the dorsal area for sensory neurons to develop correctly.
Their findings, revealed in Cell Studies, reshape our understanding of how advanced spinal circuits are established throughout embryonic growth and will inform future therapeutic methods for spinal cord restore.
The analysis was led by senior writer Samantha Butler, a professor of neurobiology on the David Geffen College of Drugs.
This can be a story of scientific curiosity -; of discovering one thing odd and attempting to grasp why it occurred. We discovered that netrin1, which we have lengthy often called a strong architect of neural circuits, has a wholly unanticipated function in organizing the spinal cord throughout early growth.”
Samantha Butler, member of the UCLA Broad Stem Cell Analysis Middle
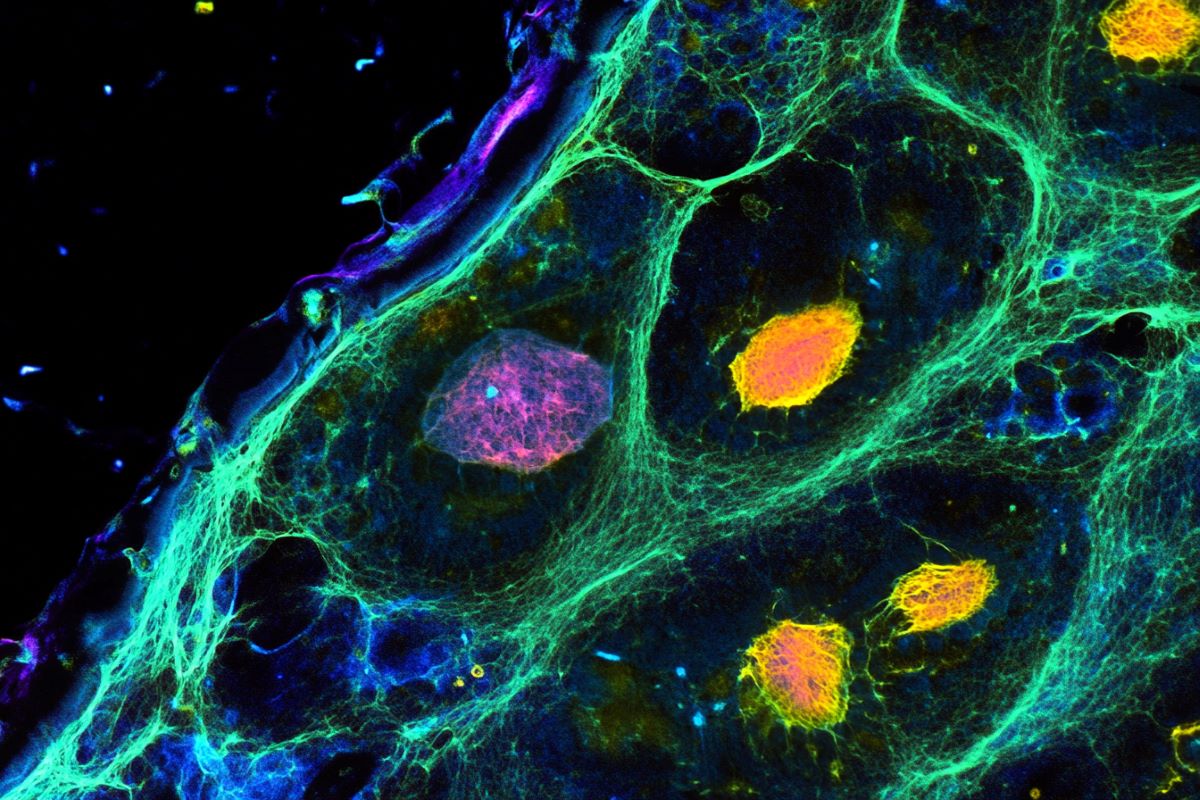
The event of the dorsal spinal cord, the place sensory inputs like contact and ache are processed, is characterised by exact compartmentalization and group. For these sensory processes to perform, particular neurons should type in fastidiously outlined areas. This exercise is orchestrated by BMP signaling, which happens solely inside the boundaries of the dorsal spinal cord.
BMP alerts should be fastidiously contained to make sure they do not unfold to different areas of the spine, disrupting the formation of different neuron sorts. The vital boundary keeper, Butler and her group found, was netrin1.
“The regional specificity of signaling molecules like BMP and netrin1 is extraordinarily essential for correct neural community formation and performance,” stated Sandy Alvarez, a graduate scholar in Butler’s lab and first writer of the research. “With out netrin1’s regulation, we might seemingly see a disorganized neural community, probably affecting how, and even when, axons attain their targets.” notice
By setting boundaries on BMP signaling, netrin1 performs a pivotal function in ensuring that sensory neurons develop within the dorsal area away from motor and interneurons within the ventral area, a division important for the right relay of sensory enter and motor output all through the physique.
In 2017, Butler and her colleagues overturned a long-standing paradigm about axon progress throughout embryonic growth. For many years, scientists had believed that axons -; skinny fibers that join cells within the nervous system -; have been attracted or repelled by steerage cues like netrin1 over lengthy distances. Butler’s analysis revealed, nonetheless, that netrin1 acts extra like a sticky adhesive floor, guiding axon progress straight alongside pathways somewhat than appearing as a distant cue.
This sudden discovery prompted Butler’s group to discover additional. In gain-of-function experiments with hen and mouse embryos, together with mouse embryonic stem cells, they launched a traceable model of netrin1 to the growing spinal cord to watch the ensuing adjustments.
Curiously, they discovered that axons had disappeared.
Alvarez initially thought one thing had gone fallacious -; that her experiments had failed. However when the outcomes repeated a number of instances over, she made the shocking connection.
“We knew that BMPs play a key function in patterning the dorsal spinal cord throughout embryonic growth, however there was nearly no scientific literature in regards to the interplay between netrin1 and BMP signaling,” Alvarez stated. “I spotted what I used to be observing was the repression of BMP exercise by netrin1 in our animal fashions.”
Utilizing a mix of genetic approaches in animal fashions, the group demonstrated that manipulating netrin1 ranges particularly altered the patterning of sure nerve cells within the dorsal spinal cord. When netrin1 ranges elevated, sure dorsal nerve cell populations disappeared; when netrin1 was eliminated, these populations expanded.
Additional bioinformatics evaluation helped set up why this was occurring: The researchers discovered that netrin1 was not directly inhibiting BMP exercise by controlling RNA translation.
“Netrin1 is essentially the most highly effective architect of neuronal circuits that I’ve ever labored with,” Butler stated. “Our subsequent endeavor will probably be to grasp how we are able to deploy netrin1 to rebuild circuitry in sufferers with nerve injury or injured spinal cords.”
Whereas the group will proceed to discover how these findings might inform potential scientific functions, together with netrin1-based therapies for neural restore, their findings might have implications past spinal cord growth. Netrin1 and BMP are additionally expressed in different organs all through the physique the place exact cell patterning is essential.
“Our outcomes recommend a have to re-evaluate how netrin1 and BMP work together in different techniques,” Alvarez stated. “This might inform our understanding of sure cell sort cancers or developmental disruptions the place BMP and netrin1 are concerned.”
Different UCLA authors embrace Sandeep Gupta, Yesica Mercado-Ayon, Kaitlyn Honeychurch, Cristian Rodriguez and Riki Kawaguchi.
The analysis was funded by the UCLA Senior Undergraduate Analysis Scholarship; the CSUN CIRM Bridges 3.0 Stem Cell Analysis & Remedy coaching program; Nationwide Institutes of Health, Nationwide Science Basis and UCLA graduate fellowships, together with help from the Eugene V. Cota-Robles, Whitcome and Hilliard Neurobiology awards; the UCLA Broad Stem Cell Analysis Middle (BSCRC) postdoctoral coaching grant; and grants from the Nationwide Institutes of Health and innovation awards from the BSCRC.
Supply:
College of California – Los Angeles Health Sciences
Journal reference:
Alvarez, S., et al. (2024). Netrin1 patterns the dorsal spinal cord by way of modulation of Bmp signaling. Cell Studies. doi.org/10.1016/j.celrep.2024.114954.


















Discussion about this post