Abstract: Scientists have efficiently reprogrammed astroglia, a kind of mind assist cell, into neurons that mimic particular interneurons essential for mind operate. By modifying the Ascl1 protein, they elevated its effectivity in changing astroglia to neuron-like cells, opening new prospects for regenerative therapies for mind issues reminiscent of epilepsy.
The engineered neurons exhibit high-frequency firing, a signature of sure interneurons important for regulating mind exercise. This work suggests astroglia may function a restore mechanism, permitting us to revive misplaced or broken mind circuits.
Key Information:
- Modified Ascl1 protein successfully converts astroglia into purposeful neurons.
- Reprogrammed neurons exhibit high-frequency firing, essential for mind circuit management.
- This method holds potential for treating situations like epilepsy by restoring neural circuits.
Supply: King’s School London
Researchers have efficiently demonstrated how astroglia – cells that assist the functioning of the mind – could be reprogrammed into cells resembling interneurons.
The analysis, revealed in Science Advances, represents not solely an essential step ahead in neuronal engineering, but in addition has very important implications for regenerative drugs, which researchers hope might be used to revive dysfunctional mind circuits like these seen in folks with epilepsy.
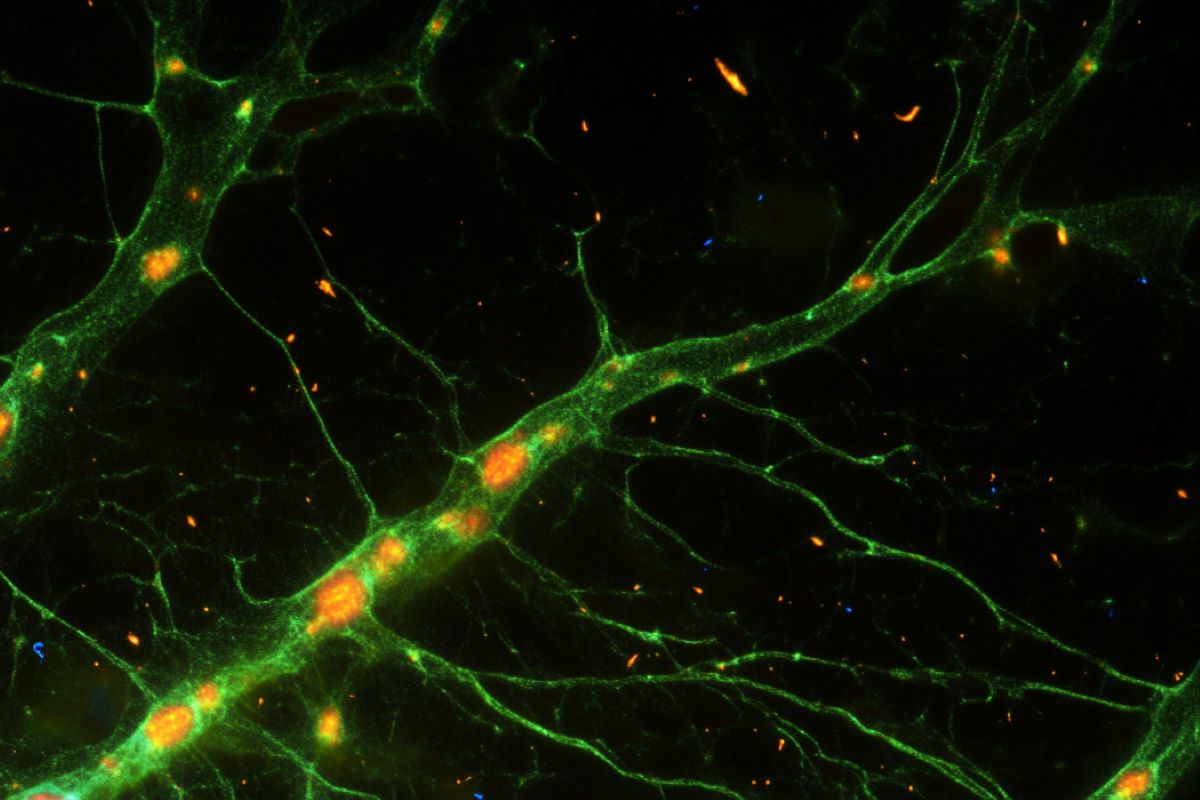
Working with mice shortly after beginning, researchers coaxed astroglia to synthesize a protein, Ascl1, that performs a key function within the improvement of the nervous system.
They discovered that when mutated, Ascl1 turned extremely environment friendly in changing astroglia into functioning neurons, rather more so than the type of the protein that’s generated naturally by the physique.
“Whereas the neurons we induced differ from these the physique creates itself, we’re excited to point out that engineered neurons can purchase extremely particular properties.
“Our findings will permit us to additional shut the hole between induced and endogenous neurons and thereby render them ever extra helpful for future translation in regenerative drugs.” Mentioned Professor Benedikt Berninger, Professor of Developmental Neurobiology at King’s IoPPN and the examine’s senior writer
The analysis group discovered that the neurons they generated displayed properties that had been much like these native to the brains they had been engaged on, together with the flexibility to fireplace at very excessive frequencies, a telltale hallmark of a selected class of interneurons that play an important function in regulating mind circuitry.
Dr Nicolás Marichal, Analysis Affiliate on the Centre for Developmental Neurobiology at King’s IoPPN and one of many examine’s lead authors stated, “This landmark examine’s success in creating neurons from astroglia breaks new floor in regenerative drugs, providing promise for the restoration of aberrant circuitry and mind operate in neurological situations.
“This work paves the way in which for additional analysis to use these findings and leverage lineage reprogramming of glia into subtype particular neurons as a brand new therapeutic avenue.”
Funding: This analysis was funded partly by Wellcome Belief, the European Analysis Council (ERC) beneath the European Union’s Horizon 2020 Analysis and Innovation Programme, and the German Analysis Basis.
About this neuroscience analysis information
Writer: Benedikt Berninger
Supply: King’s School London
Contact: Benedikt Berninger – King’s School London
Picture: The picture is credited to Neuroscience Information
Authentic Analysis: Open entry.
“Reprogramming astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site–poor Ascl1” by Benedikt Berninger et al. Science Advances
Summary
Reprogramming astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site–poor Ascl1
Mobile reprogramming of mammalian glia to an induced neuronal destiny holds the potential for restoring diseased mind circuits.
Whereas the proneural issue achaete-scute complex-like 1 (Ascl1) is broadly used for neuronal reprogramming, within the early postnatal mouse cortex, Ascl1 fails to induce the glia-to-neuron conversion, as an alternative selling the proliferation of oligodendrocyte progenitor cells (OPC).
Since Ascl1 exercise is posttranslationally regulated, right here, we investigated the results of mutating six serine phospho-acceptor websites to alanine (Ascl1SA6) on lineage reprogramming in vivo.
Ascl1SA6 exhibited elevated neurogenic exercise within the glia of the early postnatal mouse cortex, an impact enhanced by coexpression of B cell lymphoma 2 (Bcl2).
Genetic fate-mapping revealed that almost all induced neurons originated from astrocytes, whereas just a few derived from OPCs.
Many Ascl1SA6/Bcl2-induced neurons expressed parvalbumin and had been able to high-frequency motion potential firing.
Our examine demonstrates the genuine conversion of astroglia into neurons that includes subclass hallmarks of cortical interneurons, advancing our scope of engineering neuronal fates within the mind.



















Discussion about this post