DUBLIN, October 3, 2024 –( BUSINESS WIRE )– Mainstay Medical introduced at the moment that the U.S. Facilities for Illness Management and Prevention (CDC) has designated an Worldwide Classification of Illnesses, Tenth Revision, Scientific Modification (ICD-10-CM) diagnostic code particularly for multifidus dysfunction within the decrease again. The brand new diagnostic code, M62.85: dysfunction of the multifidus muscle tissues, lumbar area, is efficient October 1, 2024.
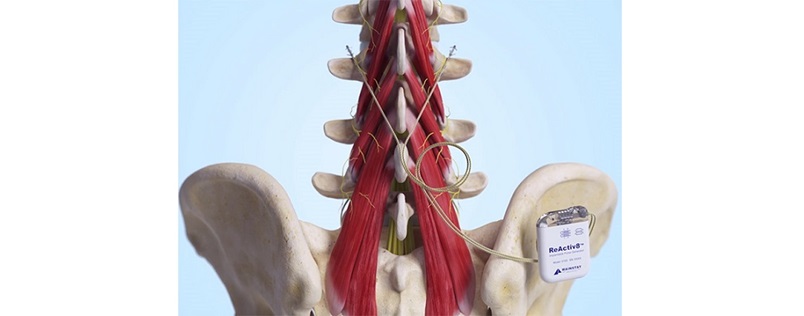
In response to an acute injury to the low again, the mind usually reduces the neural drive that prompts the multifidus muscle, leading to decreased multifidus muscle exercise. Because the multifidus muscle is a major stabilizer of the lumbar spine, this inhibition can result in joint instability and overload, exacerbating the issue and contributing to continual low again ache. The ReActiv8® Restorative Neurostimulation™ System is designed to offer electrical stimulation of the nerves activating the multifidus muscle, serving to to revive its operate and facilitate restoration from continual low again ache. ReActiv8 is the one FDA-approved therapy for the administration of intractable continual low again ache related to multifidus muscle dysfunction.
“The CDC has acknowledged multifidus dysfunction as a major explanation for continual low again ache and now supplies clinicians with a clearer path to particularly diagnose this situation. Multifidus dysfunction is a really particular illness state that doesn’t reply to palliative types of stimulation, and we’re happy that this new code clearly displays the underlying illness state,” mentioned Jason Hannon, CEO, Mainstay Medical .
About ReActiv8®
ReActiv8 is an implantable medical gadget supposed for the therapy of adults with continual intractable low again ache related to multifidus dysfunction. Multifidus dysfunction will be recognized by imaging or physiological testing in adults for whom remedies, together with analgesics and physiotherapy, have failed and for whom spinal surgical procedure will not be really helpful. ReActiv8 has acquired regulatory approvals in a number of geographies. The gadget is accessible within the European Financial Space, Australia, the UK and america.
About Mainstay Medical
Mainstay Medical is a medical gadget firm targeted on commercializing its revolutionary implantable restorative neurostimulation system, the ReActiv8, for individuals with continual, mechanically disabling low again ache. Mainstay Medical is headquartered in Dublin, Eire, with subsidiaries in Eire, america, Australia, Germany and the Netherlands.
For extra particulars: www.mainstaymedical.com .
Mainstay Ahead-Trying Statements
Statements contained on this press launch, aside from statements of historic reality, are or could also be deemed to be forward-looking statements. These forward-looking statements might embrace, however usually are not restricted to, statements relating to the Firm’s present intentions, beliefs or expectations relating to, amongst different issues, the Firm’s use of the brand new ICD-10 code and the Firm’s enterprise efforts and efficiency, outcomes, monetary situation, financing methods, product design and improvement, mental property portfolio and scope, regulatory functions and approvals and reimbursement phrases.
Ahead-looking statements contain dangers and uncertainties and usually are not ensures of future efficiency. Precise outcomes might differ materially from these described or prompt by the forward-looking statements. Numerous components might trigger outcomes and developments to vary materially from these expressed or implied by the forward-looking statements on this launch, together with the dangers and uncertainties included within the Firm’s Annual Report for the 12 months ended December 31, 2022, which ought to be learn at the side of the Firm’s public data (accessible on the Firm’s web site www.mainstaymedical.com ). Ahead-looking statements communicate solely as of the date of this launch.
The textual content of the press launch ensuing from a translation ought to on no account be thought-about official. The one model of the press launch that’s authoritative is that of the press launch in its unique language. The interpretation should all the time be in contrast with the supply textual content, which can represent precedent.
Contacts
Mainstay, Public Relations and Investor Relations:
LifeSci Advisors, LLC
Brian Ritchie
Tel. : + 1 (212) 915-2578
E mail: britchie@lifesciadvisors.com
FTI Consulting (for Eire)
Jonathan Neilan or Patrick Berkery
Tel: +353 86 602 5988
E mail: mainstay@fticonsulting.com
Mainstay Medical
Company Communications
E mail: Media@mainstaymedical.com


















Discussion about this post