Preliminary LIFEHAB Enrolled Sufferers Obtain Nociscan Exams to Assist Consider How Biomarkers within the Discs of the Lumbar Backbone Correspond With Therapy Response
LIFEHAB is a Randomized Managed Trial in Norway Evaluating Lumbar Interbody Fusion Surgical procedure With Multidisciplinary Rehabilitation
LIFEHAB Joins the NIH and Rome in Deciding on Nociscan as a Expertise to Assist Decide Which Discs to Deal with and the Expertise’s Function in Deciding on Optimum Therapy Choices
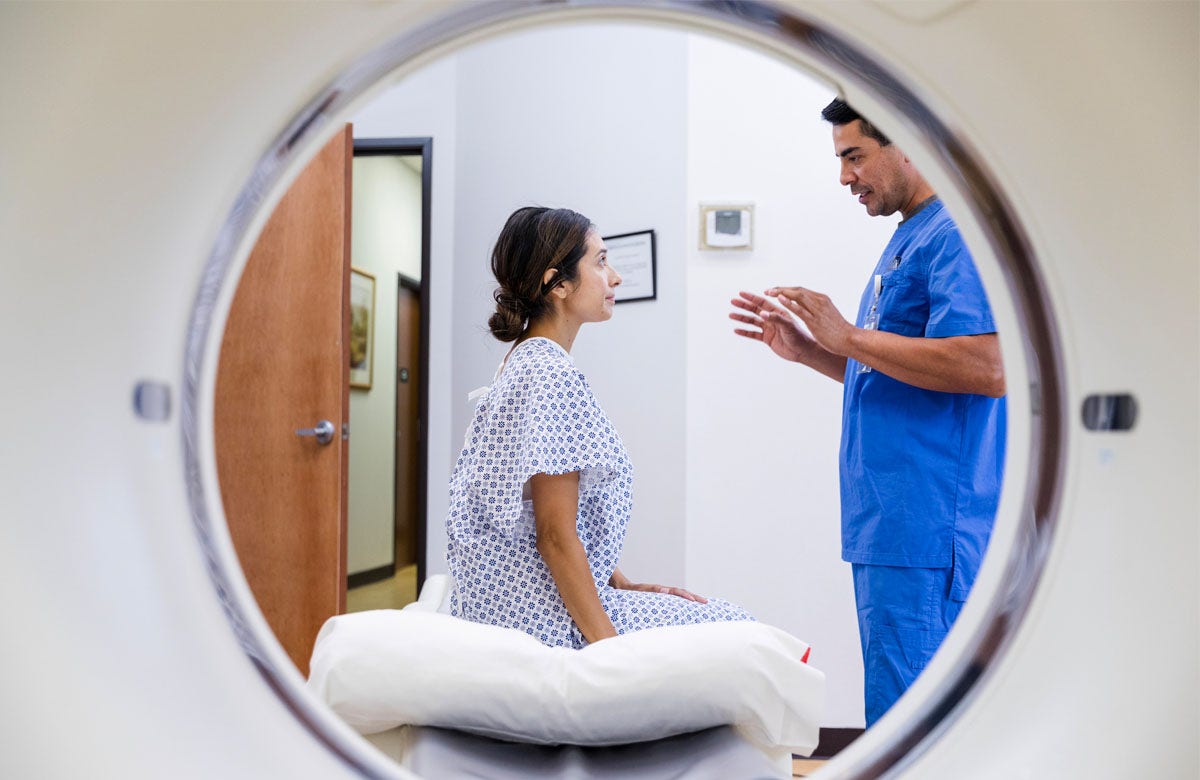
BROOMFIELD, CO, Sept. 10, 2024 (GLOBE NEWSWIRE) — Aclarion, Inc., (“Aclarion” or the “Firm”) (Nasdaq: ACON, ACONW), a healthcare expertise firm that’s leveraging biomarkers and proprietary augmented intelligence algorithms to assist physicians determine the placement of power low again ache, introduced right now the completion of the primary Nociscan exams for sufferers enrolled within the LIFEHAB Trial in Norway. The randomized managed trial learning 202 sufferers with low again ache higher than 1 12 months in length will examine remedy outcomes between lumbar interbody fusion surgical procedure and multidisciplinary rehabilitation. Nociscan was chosen to be used within the examine to assist consider how magnetic resonance spectroscopy (MRS) biomarkers can determine affected person enchancment following remedy.
Affected person enrollment in LIFEHAB started within the second quarter of 2024. The preliminary enrolled sufferers started finishing Nociscan exams within the final week of August. To this point six (6) sufferers have accomplished their Nociscan exams.
“We’re excited to see the LIFEHAB Trial progressing on schedule and look ahead to the outcomes of the examine and to the function we count on Nociscan knowledge to play in not solely serving to physicians decide which discs to deal with however in doubtlessly serving to to foretell which remedy choice is perfect for a specific affected person,” stated Brent Ness, Aclarion CEO. “Though we speak incessantly about driving Nociscan to plain of take care of medical use, the analysis market is proving to be an early adopter of our expertise.”
Aclarion is presently awaiting the outcomes of a accomplished trial in Rome the place Nociscan expertise was utilized in a examine evaluating regenerative applied sciences in addition to the outcomes of two NIH research evaluating the function of Nociscan in treating power low again ache.
“We see Nociscan shifting at an accelerated tempo of adoption within the analysis market and look ahead to saying extra partnerships as we proceed to drive Nociscan to what we count on to be the gold normal for figuring out which discs to deal with in analysis protocols on low again ache,” stated Ryan Bond, Chief Technique Officer of Aclarion.
Aclarion believes the outcomes of the LIFEHAB trial, mixed with different compelling proof, marks a key step towards reaching profitable reimbursement in a single-payer nationwide medical insurance system like Norway.
LIFEHAB is the newest customer-sponsored medical trial involving Nociscan and the Firm appears to be like ahead to saying further partnerships as Nociscan expands its adoption within the analysis market.
For extra data on the LIFEHAB Trial:
https://basic.clinicaltrials.gov/ct2/present/NCT06169488
About Aclarion, Inc.
Aclarion is a healthcare expertise firm that leverages Magnetic Resonance Spectroscopy (“MRS”), proprietary sign processing methods, biomarkers, and augmented intelligence algorithms to optimize medical therapies. The Firm is first addressing the power low again ache market with Nociscan, the primary, evidence-supported, SaaS platform to noninvasively assist physicians distinguish between painful and nonpainful discs within the lumbar spine. By a cloud connection, Nociscan receives magnetic resonance spectroscopy (MRS) knowledge from an MRI machine for every lumbar disc being evaluated. Within the cloud, proprietary sign processing methods extract and quantify chemical biomarkers demonstrated to be related to disc ache. Biomarker knowledge is entered into proprietary algorithms to point if a disc could also be a supply of ache. When used with different diagnostic instruments, Nociscan gives important insights into the placement of a affected person’s low again ache, giving physicians readability to optimize remedy methods. For extra data, please go to www.aclarion.com
Ahead Trying Statements
This press launch accommodates forward-looking statements inside the that means of the Personal Securities Litigation Reform Act of 1995, Part 27A of the Securities Act of 1933 and Part 21E of the Securities Alternate Act of 1934 in regards to the Firm’s present expectations about future outcomes, efficiency, prospects, and alternatives. Statements that aren’t historic info, similar to “anticipates,” “believes” and “expects” or comparable expressions, are forward-looking statements. These forward-looking statements are based mostly on the present plans and expectations of administration and are topic to a lot of uncertainties and dangers that might considerably have an effect on the Firm’s present plans and expectations, in addition to future outcomes of operations and monetary situation. These and different dangers and uncertainties are mentioned extra absolutely in our filings with the Securities and Alternate Fee. Readers are inspired to assessment the part titled “Threat Elements” within the Firm’s Annual Report on Kind 10-Ok for the 12 months ended December 31, 2023, in addition to different disclosures contained within the Prospectus and subsequent filings made with the Securities and Alternate Fee. Ahead-looking statements contained on this announcement are made as of this date and the Firm undertakes no obligation to publicly replace or revise any forward-looking statements, whether or not on account of new data, future occasions or in any other case.
Investor Contacts:
Kirin M. Smith
PCG Advisory, Inc.
646.823.8656
ksmith@pcgadvisory.com
Media Contacts:
Jodi Lamberti
SPRIG Consulting
612.812.7477
jodi@sprigconsulting.com



















Discussion about this post