Abstract: A brand new experimental most cancers drug might ease cognitive difficulties for these with Rett syndrome, a uncommon autism-linked dysfunction, by enhancing mind cell capabilities. The drug, ADH-503, improves the exercise of microglia, that are essential for sustaining neural networks.
Researchers discovered that wholesome microglia restored synapse operate in mind organoids mimicking Rett syndrome. This breakthrough suggests potential therapies for Rett syndrome and different neurological circumstances.
Key Details:
- ADH-503, an experimental most cancers drug, might assist cognitive operate in Rett syndrome.
- The drug enhances microglia exercise, bettering synapse operate in mind organoids.
- This analysis might result in therapies for varied neurological circumstances.
Supply: UCSD
An experimental most cancers drug might make pondering simpler for people with Rett syndrome, a uncommon dysfunction linked to autism, in accordance with new analysis from the College of California San Diego — a discovery that might result in therapies for sufferers with different neurological circumstances.
The findings, revealed July 25 in Stem Cell Experiences, spotlight the function of microglia — a kind of white blood cell discovered within the central nervous system — within the formation of the human mind.

Whereas such cells have been higher studied in neurodegenerative issues like Alzheimer’s illness, amyotrophic lateral sclerosis (ALS) and a number of sclerosis, “little or no info has existed on their function in early levels of neural growth” as a result of entry to fetal tissue is restricted, stated Pinar Mesci, Ph.D., the research’s lead researcher.
Now employed elsewhere, she accomplished work on the challenge whereas on the college.
In a bid to higher perceive their operate, Mesci as an alternative used mind organoids — “mini brains,” primarily, that mimic the creating mind of an embryo — grown from skin-derived stem cells of consenting sufferers.
Such organoids had been created from people with Rett syndrome — a dysfunction primarily present in females that options lack of speech, purposeful use of arms, mobility and muscle tone, amongst different signs — in addition to from neurotypical people.
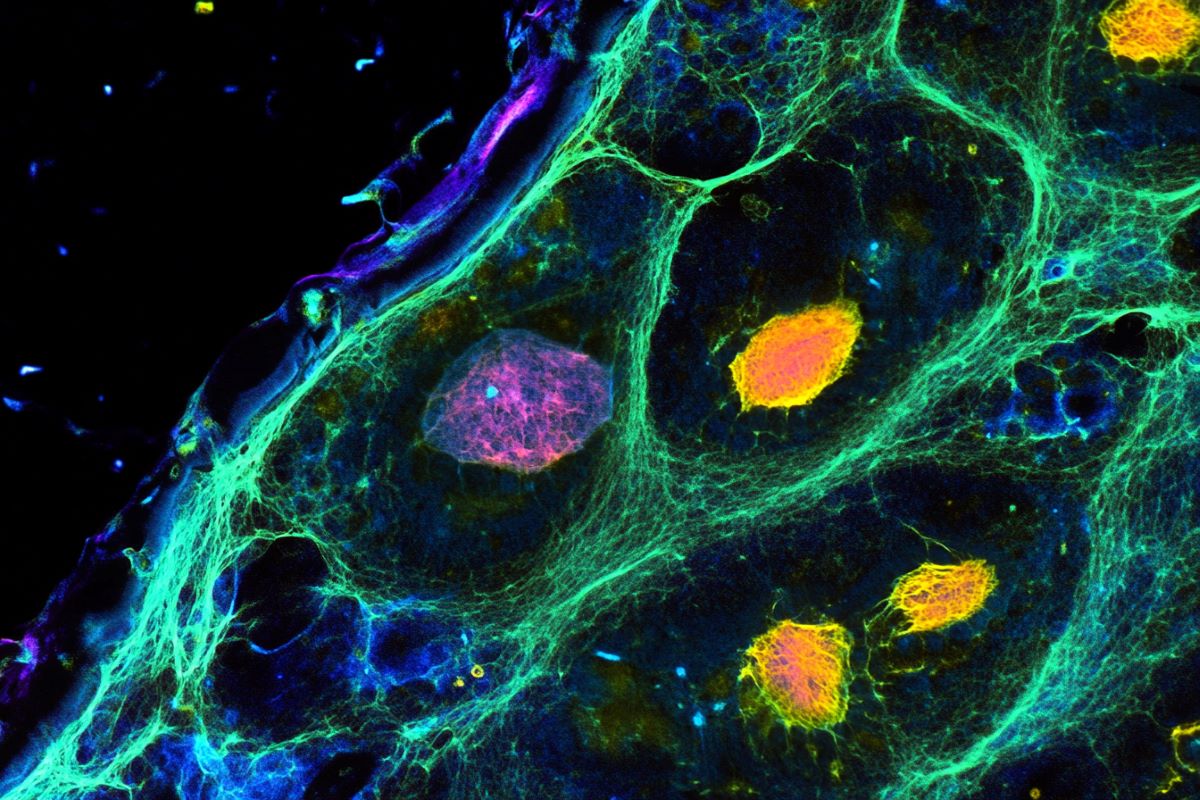
Mesci then added wholesome microglia to the Rett syndrome mind organoids and located that the functioning of synapses — the place neurons join and talk — was “rescued.”
This occurred as a result of restoration of phagocytosis, a course of by which microglia — generally known as the “janitors” of the central nervous system — ingest and destroy international substances like micro organism and lifeless cells, preserving the mind and spinal cord tidy. The method additionally entails “pruning” of synapses, which optimizes mind operate.
Researchers additionally discovered that the synapses of typical neurons skilled impaired functioning when Rett syndrome microglia had been launched, additional confirming the function of the immune cell in mind operate and growth.
“If the mind’s ‘janitors’ are usually not working, issues begin to come up,” stated UC San Diego College of Medication professor Alysson Muotri, Ph.D., senior creator and director of the college’s Sanford Stem Cell Institute’s Built-in House Stem Cell Orbital Analysis Heart.
Defective microglia make cognition even tougher for Rett syndrome sufferers, who already take care of fewer and impaired synapses and dysfunctional astrocytes because of a lack of operate within the MECP2 gene, implicated in different sorts of neurodevelopmental circumstances as effectively.
Microglia with lack of MECP2 operate “are usually not pretty much as good at pruning synapses and shaping the neural community — they don’t do an excellent job,” Muotri stated.
The crew then examined a battery of present medication on the microglia, to see if any may restore phagocytosis. They discovered one: ADH-503, often known as GB1275 — an experimental oral pancreatic most cancers remedy that additionally reduces the variety of immune-supressing cells that enter a tumor. The drug serves as a regulator of CD11b, a protein concerned in phagocytosis, amongst different processes.
Different research on Rett syndrome have highlighted potential therapeutic targets. However none to this point have recognized a possible therapy involving human microglial cells.
By the point Rett syndrome sufferers are recognized, it’s too late to restore and never presently attainable to interchange defective neurons, the first subject within the illness. “However by specializing in different cell varieties — and probably discovering medication that enhance how they work — we would enhance the surroundings for these neurons and ease functioning for sufferers,” Mesci stated. “That’s what I’m enthusiastic about.”
Jonathan Kipnis, Ph.D., professor of pathology, immunology, neurology, neuroscience and neurosurgery at Washington College College of Medication in St. Louis and director of its Mind Immunology and Glia Heart, stated the brand new analysis “properly demonstrates” microglia as a possible therapeutic goal in Rett syndrome.
“I hope this work will ‘transfer the needle’ and convey the Rett neighborhood again to neuroimmunology,” Kipnis stated.
“Understanding neuro-immune interactions on this advanced illness might not solely present new insights into the illness biology, but additionally develop novel approaches to attenuate its development.”
The analysis represents the primary profitable integration of human microglia into Rett syndrome mind tissues in vitro — a mannequin that will show superior to mouse fashions.
The researchers hope the research “opens doorways for therapies,” not just for these with Rett syndrome, however for these with different neurodevelopmental and neurodegenerative issues during which microglia play a task.
“That’s my want,” Mesci stated, “that we will enhance high quality of life.”
Co-authors of the research embrace Christopher LaRock, with the Division of Pediatrics on the College California San Diego College of Medication and Skaggs College of Pharmacy and Pharmaceutical Sciences; Jacob J. Jeziroski, Natalia Chermount, Tomoka Ozaki, Aurian Saleh, Cedric E. Snethlage, Sandra Sanchez, Gabriela Goldberg, Intelligent A. Trujillo and Kinichi Nakashima, with the College of California San Diego College of Medication and Division of Pediatrics at Rady Youngsters’s Hospital San Diego, and the Division of Mobile & Molecular Medication; Hideyuki Nakashima, with the Division of Stem Cell Biology and Medication at Kyushu College’s Graduate College of Medication; Adriano Ferrasa, with the Experimental Multiuser Labratory on the Graduate Program in Health Sciences on the College of Medication at Pontifícia Universidade Católica do Paraná in Curitiba, Paraná, Brazil, in addition to the Division of Informatics on the Universidade Estadual de Ponta Grossa in Ponta Grossa, Parana, Brazil; Roberto H. Herai, with the Experimental Multiuser Labratory on the Graduate Program in Health Sciences on the College of Medication at Pontifícia Universidade Católica do Paraná in Curitiba, Paraná, Brazil, and the Analysis Division at Lico Kaesemodel Institute in Curitiba, Paraná, Brazil; and Victor Nizet, with the Division of Pediatrics on the College California San Diego College of Medication and Skaggs College of Pharmacy and Pharmaceutical Sciences.
Funding: This work was made attainable partly by the California Institute for Regenerative Medication (CIRM) Main Amenities grant (FA1-00607) to the Sanford Consortium for Regenerative Medication. Muotri is supported by the Nationwide Institutes of Health (NIH) R01MH107367, R01HD107788, R01NS105969 and R01NS123642, and a grant from the Worldwide Rett Syndrome Basis (IRSF).
This work was additionally partially funded by the IRSF Innovation Award granted to Mesci (grant No. 3905).
Herai is funded by Fundação Araucária (grant No. FA09/2016). This work was additionally partially funded by AMED (JP22mg1310008), an Intramural Analysis Grant (3-9) for Neurological and Psychiatric Problems of NCNP grant to Nakashima and a Japan Society for the Promotion of Science (JSPS) KAKENHI (JP22K15201) to Nakashima.
This publication contains knowledge generated on the UC San Diego IGM Genomics Heart using an Illumina NovaSeq 6000 that was bought with funding from an NIH SIG grant (No. S10 OD026929).
Disclosures: Muotri is a co-founder and has an fairness curiosity in TISMOO, an organization devoted to genetic evaluation and human mind organogenesis specializing in therapeutic functions personalized for autism spectrum dysfunction and different neurological issues with genetic origins.
The phrases of this association have been reviewed and accredited by the College of California San Diego in accordance with its conflict-of-interest insurance policies. The authors have a patent utility within the works associated to this publication.
About this autism and neuropharmacology analysis information
Creator: Danielle Lewis
Supply: UCSD
Contact: Danielle Lewis – UCSD
Picture: The picture is credited to Muotri Lab/ UC San Diego Health Sciences
Authentic Analysis: Open entry.
“Human microglial cells as a therapeutic goal in a neurodevelopmental illness mannequin” by Pinar Mesci et al. Stem Cell Experiences
Summary
Human microglial cells as a therapeutic goal in a neurodevelopmental illness mannequin
Highlights
- MECP2 is implicated in microglial capabilities together with phagocytosis
- Human microglia are implicated in synapse formation
- CD11b agonist ADH-503 restored phagocytosis and synaptic defects and elevated survival
- Human microglia could be promising therapeutic targets for neurological circumstances
Abstract
Though microglia are macrophages of the central nervous system, their involvement shouldn’t be restricted to immune capabilities. The roles of microglia throughout growth in people stay poorly understood because of restricted entry to fetal tissue.
To know how microglia can impression human neurodevelopment, the methyl-CpG binding protein 2 (MECP2) gene was knocked out in human microglia-like cells (MGLs). Disruption of the MECP2 in MGLs led to transcriptional and purposeful perturbations, together with impaired phagocytosis.
The co-culture of wholesome MGLs with MECP2-knockout (KO) neurons rescued synaptogenesis defects, suggesting a microglial function in synapse formation.
A focused drug screening recognized ADH-503, a CD11b agonist, restored phagocytosis and synapse formation in spheroid-MGL co-cultures, considerably improved illness development, and elevated survival in MeCP2-null mice.
These outcomes unveil a MECP2-specific regulation of human microglial phagocytosis and determine a novel therapeutic therapy for MECP2-related circumstances.

















Discussion about this post