SALT LAKE CITY, July 9, 2024 /PRNewswire/ — DiscGenics, Inc., a privately held, late-stage medical, biopharmaceutical firm growing allogeneic, cell-based, regenerative therapies for musculoskeletal degeneration, right now introduced publication of ends in the Worldwide Journal of Backbone Surgical procedure from the mixed Section I/Section II, first-in-human medical research of an allogeneic disc progenitor cell remedy (IDCT or rebonuputemcel) for painful lumbar degenerative disc illness (DDD).
The research met the first security and efficacy endpoints, exhibiting {that a} single intradiscal injection of high-dose IDCT (9,000,000 cells/mL) safely will increase disc quantity and produces statistically important, clinically significant enhancements in again ache, incapacity, and high quality of life out to 2 years post-injection in sufferers with lumbar disc degeneration.
“The outcomes from this research reveal IDCT’s potential to securely and successfully scale back ache related to DDD whereas additionally producing a regenerative impact inside the degenerating disc. MRI picture evaluation of disc quantity indicated the potential to halt and presumably reverse the development of the illness,” mentioned Matthew F. Gornet, M.D., lead creator, Board Licensed Backbone Surgeon at The Orthopedic Heart of St. Louis, and high enroller within the IDCT research. “I’ve been a training spine surgeon for greater than 30 years and been concerned in over 35 FDA medical trials, and the affected person outcomes from this research are very promising.”
Within the FDA-allowed, potential, randomized, double-blinded, vehicle- and placebo-controlled, multicenter research, 60 sufferers with symptomatic, single-level lumbar DDD had been randomized to obtain single intradiscal injections of both low-dose cells (n = 20), high-dose cells (n = 20), automobile alone (n = 10), or placebo (n = 10). The first endpoint was imply visible analog scale (VAS) ache enchancment >30% at 52 weeks. Incapacity and high quality of life had been evaluated by way of Oswestry Incapacity Index (ODI) and EQ-5D, respectively. Disc quantity was radiologically assessed. Adversarial occasions (AEs), no matter whether or not they had been associated to therapy, had been reported. Sufferers had been assessed at baseline and at 4, 12, 26, 52, 78, and 104 weeks put up therapy.
At Week 52, the first research interval, the high-dose group had a imply VAS proportion lower from baseline (−62.8%, P = 0.0005), attaining the endpoint of again ache enchancment >30%; the imply change was additionally considerably larger than the minimal clinically vital distinction (MCID) of a 20-point lower (−42.8, P = 0.001). This medical enchancment was maintained at Week 104. As well as, the high-dose group had clinically significant, statistically important enhancements in ODI and EQ-5D by 12 weeks. Medical enchancment was sustained at 26 weeks, 52 weeks, 78 weeks, and 104 weeks following a single intradiscal injection. Solely the high-dose group had a big change in disc quantity, with imply will increase of 249.0 mm3 (P = 0.028) at 52 weeks and 402.1 mm3 (P = 0.028) at 104 weeks. Total, a minority of sufferers (18.3%) reported AEs that had been extreme, with the best proportion being reported within the placebo group. In the course of the course of the trial, 6.7% of sufferers skilled critical AEs, all occurring within the automobile (n = 1) or placebo (n = 3) teams, none had been therapy associated.
“For the reason that inception of DiscGenics, we’ve got seen constant proof of security and the regenerative potential of the distinctive disc cell inhabitants in IDCT to deal with disc degeneration,” mentioned Kevin T. Foley, MD, Chief Medical Officer of DiscGenics and Chairman of Semmes-Murphey Neurologic & Backbone Institute. “Our early fundamental science research, our demonstrated capacity to securely use human cells in 14 totally different pre-clinical animal research carried out within the U.S. and Japan, and not too long ago, knowledge from our first-in-human security and patient-reported outcomes printed on this IJSS manuscript, all assist the notion that this cell has the potential to securely regenerate the intervertebral disc from the inside-out.”
DDD is a persistent and progressive situation the place the intervertebral disc breaks down and causes ache and incapacity. It accounts for almost 40% of persistent low again ache instances within the U.S., a critical medical situation that impacts 12-30% of U.S. adults at a given time and is estimated to price the U.S. healthcare system over $100 billion every year, creating a substantial burden on the financial system and particular person sufferers.
“The numerous and sturdy outcomes from this research reveal the unimaginable potential of IDCT to vary the paradigm of take care of sufferers with DDD, a situation with restricted therapy choices,” mentioned Flagg Flanagan, Chief Govt Officer and Chairman of the Board for DiscGenics.” We’re excited by the momentum the publication of those research outcomes provides, as we anticipate to provoke Section III medical research of this novel remedy within the U.S. imminently.”
About IDCT
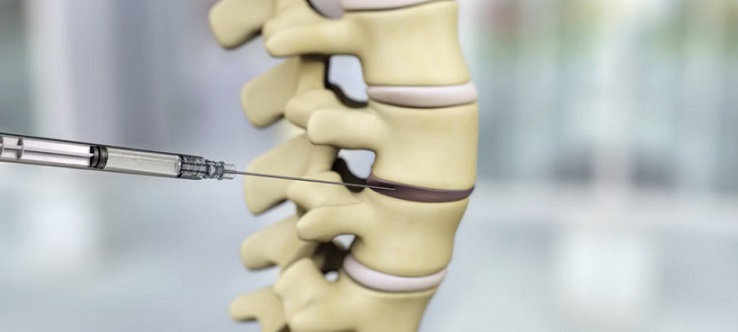
IDCT (injectable disc cell remedy, or rebonuputemcel) is a standalone, single-injection biologic therapy designed to halt development of symptomatic lumbar disc degeneration and regenerate the disc from the within out. The lively ingredient (Drug Substance) in IDCT is a stay, manufactured progenitor cell inhabitants derived from donated grownup human intervertebral disc tissue. These cells are enriched and expanded into Discogenic Cells by way of a multistep manufacturing course of in a extremely managed surroundings below present good manufacturing practices (cGMP) that ends in important proliferation and phenotypic modifications to the cells. On the completion of the manufacturing course of, the Discogenic Cells are subjected to intensive testing prior to make use of, together with identification, purity, efficiency, and security evaluations. The Discogenic Cells are then blended with a viscous Sodium Hyaluronate Resolution and excipients to generate IDCT, the Remaining Drug Product. IDCT is cryopreserved and maintained as particular person “off-the-shelf” doses for administration by way of percutaneous injection into the intervertebral disc in an outpatient setting. IDCT has been granted regenerative drugs superior remedy (RMAT) and Quick Monitor designations by the U.S. Food and Drug Administration.
Disclaimer: IDCT is an investigational product that’s below growth by DiscGenics and has not been authorised by the FDA or every other regulatory company for human use.
About DiscGenics
DiscGenics is a privately held, late-stage medical biopharmaceutical firm growing allogeneic, cell-based regenerative therapies for musculoskeletal degeneration. It’s lead product candidate, IDCT (injectable disc cell remedy, or rebonuputemcel), is a standalone, single-injection biologic therapy designed to halt development of lumbar disc degeneration and regenerate the disc from the within out. DiscGenics can be growing a follow-on allogeneic cell platform to allow new musculoskeletal indications. To additional growth of those distinctive therapies, and to keep up management over compliance, price, and manufacturing timelines, DiscGenics has constructed and validated an in-house scalable, allogeneic cell manufacturing course of and cGMP facility at its headquarters in Salt Lake Metropolis, Utah. For extra info, go to discgenics.com.
SOURCE DISCGENICS, INC.




















Discussion about this post