A current evaluation printed within the journal npj Digital Medication examines the present classes of surgical robots which were authorized by the USA Food and Drug Administration (FDA) primarily based on Ranges of Autonomy in Surgical Robotics (LASR).
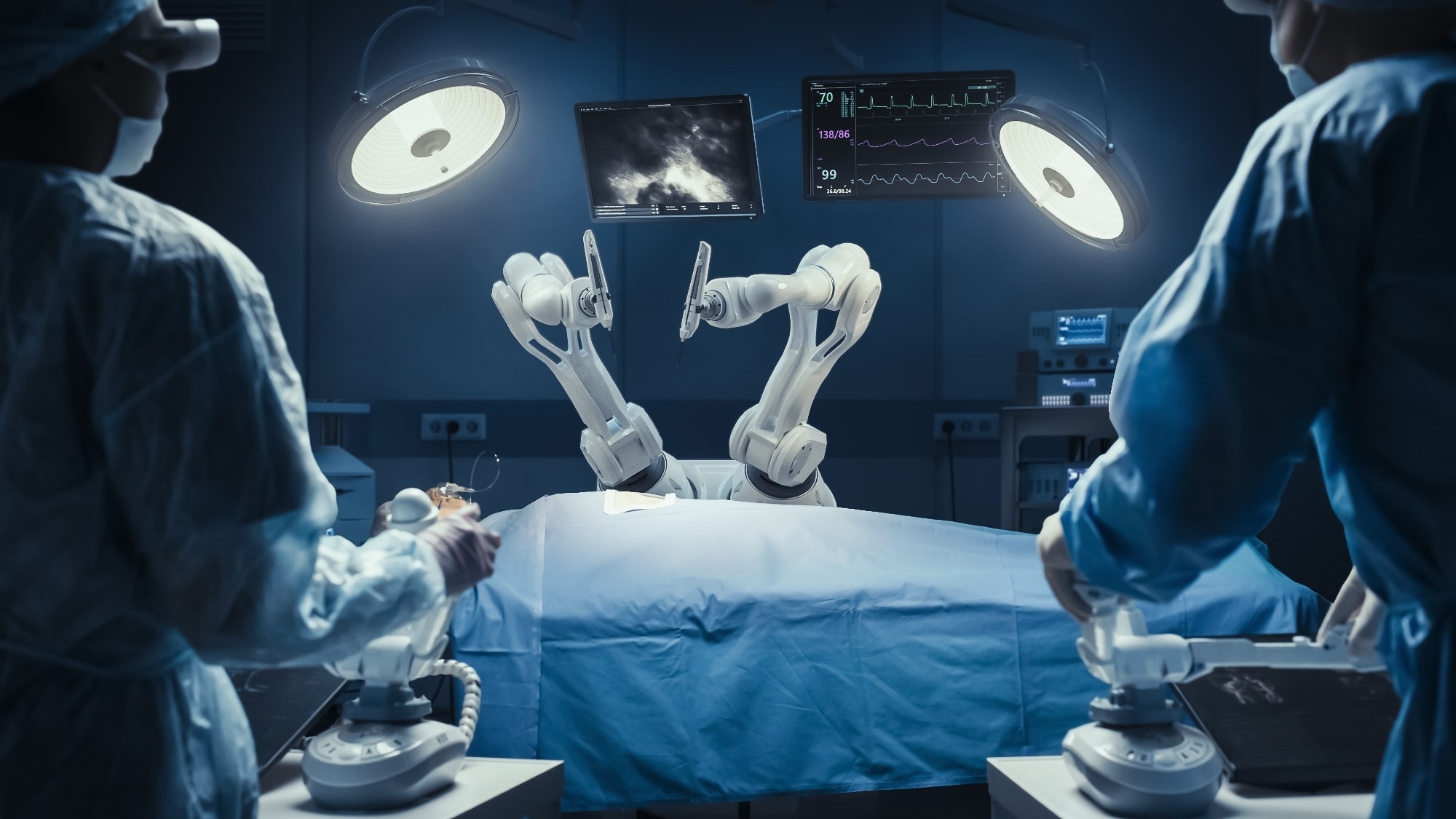
Research: Ranges of autonomy in FDA-cleared surgical robots: a scientific evaluation. Picture Credit score: Gorodenkoff / Shutterstock.com
About surgical robots
The primary surgical robotic to obtain FDA clearance was the Automated Endoscopic System for Optimum Positioning (AESOP) in 1993. By the 510(okay) Premarket Notification pathway, AESOP was assigned reasonable threat and categorised as a Class II surgical machine.
In comparison with the da Vinci Surgical System by Intuitive Surgical, through which the surgeon makes use of the robotic arm to increase his attain, fashionable surgical robots are extra advanced and autonomous. For instance, the TSolution One is each the planner and operator, whereas the surgeon is accountable primarily for supervising the instrument.
Up to now, there stays no outlined platform to categorise these completely different programs. At present, the one strategy to classify these devices is by utilizing the system utilized for industrial robots and self-driving autos.
Since 2015, the FDA has advocated for the time period “robotically-assisted surgical units” to emphasise that each one cleared programs haven’t any robotic autonomy, as they require the surgeon’s direct and steady management. The surgeon is totally liable for the protection of the process.”
The automation of surgical robots is quickly progressing, as is dependence on synthetic intelligence, particularly machine studying (ML). As these programs proceed to advance, issues concerning the obligation for automated surgical procedures have emerged, in addition to whether or not the surgeon or robotic might be thought-about accountable. It is usually essential to find out which competencies are required for extra coaching earlier than these instruments can be utilized.
In regards to the research
The authors of the present research emphasize the necessity for uniform definitions and requirements for surgical robotics throughout regulatory, requirements, and medical organizations and our bodies. The present research additionally examines the relative contribution of the surgeon and machine throughout surgical procedures.
The evaluation included all Class II surgical robots authorized by the FDA since 2015. Every robotic was examined utilizing LASR standards for autonomy classification.
What did the research present?
A complete of 49 distinctive surgical robots had been recognized, 86% of which had been Stage 1 programs operated constantly by the surgeon and are in any other case known as ‘Robotic Help.’ 4 Stage 2 ‘Job Autonomy’ programs, which had been designed by means of programming to carry out a selected surgical activity autonomously, had been additionally recognized.
Three Stage 3 programs, presently probably the most autonomous FDA-approved programs, had been recognized. These programs exhibit ‘Conditional Autonomy,’ as they will plan the process if offered with particular affected person particulars. No Stage 4 or Stage 5 surgical robots had been discovered; nevertheless, the researchers talk about the potential of sudden drastic shifts in autonomy with comparatively minor technological advances.
Predicate creep – a repetitive cycle of know-how adjustments between 510(okay) clearances that will outcome within the sudden introduction of units with excessive ranges of complexity – has been recognized in surgical robotics.”
The primary 15 robotic programs obtained clearance earlier than 2015 and had been subsequently authorized for extra capabilities. Thereafter, 24 robots had been launched from Stage 1 to Stage 3. All through this era, activity automation elevated, with principally Stage 2 robots launched since 2017.
When categorised by the regulatory system, about 90% of surgical robots obtained 510(okay) clearance, whereas the remaining programs had been authorized by means of the De Novo pathway. Scientific testing knowledge was equipped for regulatory approval in 19 circumstances, together with all Stage 3, 75% of Stage 2, and 30% of Stage 1 robots.
Two of the robots claimed to have ML-enabled capabilities on their web sites; nevertheless, their FDA summaries lacked proof of those claims.
When examined by specialty, orthopedic assistive robots concentrating on the spine, knee, and hip comprised about 33% of all new programs. One of many three superior Stage 3 programs was in orthopedics for bone milling. Robotic programs for urology, basic surgical procedure, and neurosurgery had been additionally recognized.
Conclusions
Sooner or later, LASR-based frameworks shall be important to make sure that extra autonomous units meet approval standards emphasizing scientific security. One other risk is that each one future advances in autonomy will enter Class III or high-risk units, thus making premarket approval necessary.
Though this will likely inhibit speedy innovation, these processes will be certain that stringent security requirements are adopted within the approval of those novel units. Importantly, due to these prolonged approval processes, the price and period of those approval processes can also improve.
The usage of ML may trigger vital errors to emerge by repetitively strengthening biased decision-making primarily based on coaching knowledge. This might turn into notably regarding when automated duties are carried out.
The surgical setting is vital to decision-making and threat dedication. Due to this fact, further analysis is required to contemplate the scientific state of affairs and the robotics system’s stage of autonomy when figuring out threat.
Implementing regulatory frameworks that acknowledge various ranges of autonomy in surgical robots might assist guarantee their secure and efficient integration into surgical observe.”
Journal reference:
- Lee, A., Baker, T. S., Bederson, J. B., & Rapaport, B. I. (2024). Ranges of autonomy in FDA-cleared surgical robots: a scientific evaluation. npj Digital Medication. doi:10.1038/s41746-024-01102-y.


















Discussion about this post