December 5, 2024 – OrthoSpineNews –

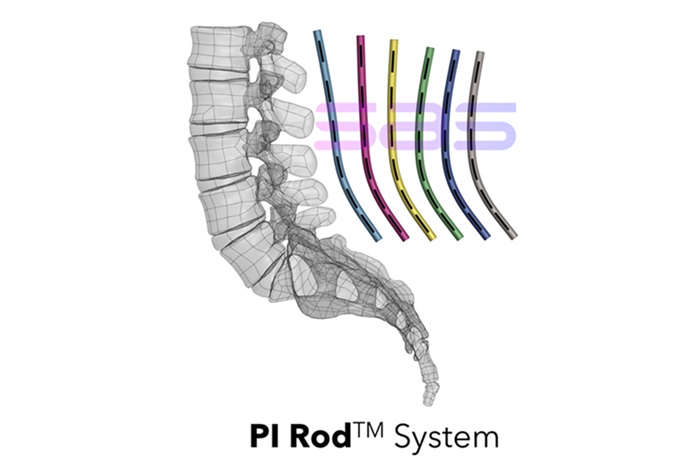
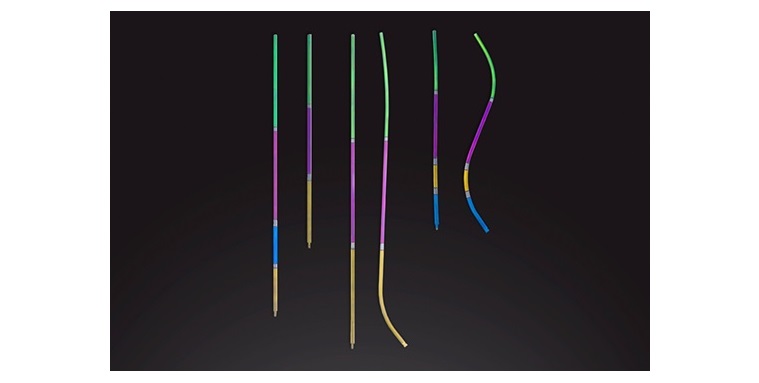
Spinal Alignment Options (S.A.S) is proud to announce that the U.S. Food and Drug Administration (FDA) has granted 510(ok) clearance (K242350) for the Pelvic Incidence (PI) Rod System, marking a pivotal development in spinal reconstruction know-how. For particulars, please go to the FDA hyperlink: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K242350
The PI RodTM System is engineered to be suitable with the Medtronic CD Horizon™ SOLERA™ Spinal System. It gives progressive, reproducible posterior lumbar and sacral fixation options for a complete vary of lumbosacral pathologies, together with degenerative disc illness, spondylolisthesis, trauma, spinal stenosis, segmental kyphosis, tumors, pseudarthrosis, and failed earlier lumbar fusions. The system is obtainable in each titanium alloy and cobalt chrome to make sure optimum outcomes tailor-made to every affected person’s wants.
“The FDA’s 510(ok) clearance of our PI RodTM System underscores our unwavering dedication to enhancing affected person care via the first-ever off-the-shelf implants designed to enhance spinal alignment,” mentioned Dr. Bassel Diebo, President and CEO of Spinal Alignment Options. “This clearance represents a transformative milestone in surgical planning, using data-driven alignment ideas. Supported by our top-tier spinal alignment engineers, Knowledge Analytics and Synthetic Intelligence, our mission is to revolutionize spinal look after degenerative circumstances, offering reproducible constructs that lastly tackle the enduring subject of short-segment flatbacks.”


















Discussion about this post