Abstract: Researchers have developed a technique to rework pores and skin cells into mind neurons, capturing the growing old results essential for learning late-onset Alzheimer’s illness. This system precisely mimics Alzheimer’s hallmarks, like amyloid beta buildup, in lab-derived neurons, permitting for detailed evaluation.
The research recognized retrotransposable components within the genome that change exercise with age, suggesting new therapy methods. Medicine focusing on these components confirmed promise in decreasing Alzheimer’s results, emphasizing the significance of early intervention.
Key Details:
- Researchers remodeled pores and skin cells into neurons to review late-onset Alzheimer’s.
- The research discovered retrotransposable components play a job in Alzheimer’s development.
- The drug lamivudine lowered Alzheimer’s results in lab-grown neuron fashions.
Supply: WUSTL
Researchers at Washington College Faculty of Drugs in St. Louis have developed a solution to seize the consequences of growing old within the growth of Alzheimer’s illness. They’ve devised a technique to review aged neurons within the lab and not using a mind biopsy, an development that would contribute to a greater understanding of the illness and new therapy methods.
The scientists remodeled pores and skin cells taken from sufferers with late-onset Alzheimer’s illness into mind cells known as neurons. Late-onset Alzheimer’s develops progressively over many a long time and solely begins to point out signs at age 65 or older.

For the primary time, these lab-derived neurons precisely reproduced the hallmarks of one of these dementia, together with the amyloid beta buildup, tau protein deposits and neuronal cell loss of life.
By learning these cells, the researchers recognized points of cells’ genomes — known as retrotransposable components, which change their exercise as we age — within the growth of late-onset Alzheimer’s illness. The findings counsel new therapy methods focusing on these elements.
The research seems Aug. 2 within the journal Science.
“Sporadic, late-onset Alzheimer’s illness is the commonest kind of Alzheimer’s illness, representing greater than 95% of circumstances,” mentioned senior writer Andrew Yoo, PhD, a professor of developmental biology.
“It has been very tough to review within the lab as a result of complexity of the illness stemming from numerous danger elements, together with growing old as an necessary contributor. Till now, we didn’t have a solution to seize the consequences of growing old within the cells to review late-onset Alzheimer’s.”
Up to now, animal research of Alzheimer’s illness have, by necessity, centered on mice with uncommon genetic mutations recognized to trigger inherited, early-onset Alzheimer’s in youthful individuals — a technique that has make clear the situation however differs from illness growth for the overwhelming majority of sufferers with the sporadic, late-onset type. To extra faithfully recapitulate the illness within the lab, Yoo’s staff turned to an strategy known as mobile reprogramming.
The strategy to rework simply obtained human pores and skin cells from residing sufferers instantly into neurons makes it doable to review Alzheimer’s results on the mind with out the chance of a mind biopsy and in a method that retains the results of the affected person’s age on the neurons.
Previous work by Yoo and his colleagues, who pioneered this transformation approach utilizing small RNA molecules known as microRNAs, has centered on understanding the event of Huntington’s illness — an inherited neurological situation that usually exhibits adult-onset signs.
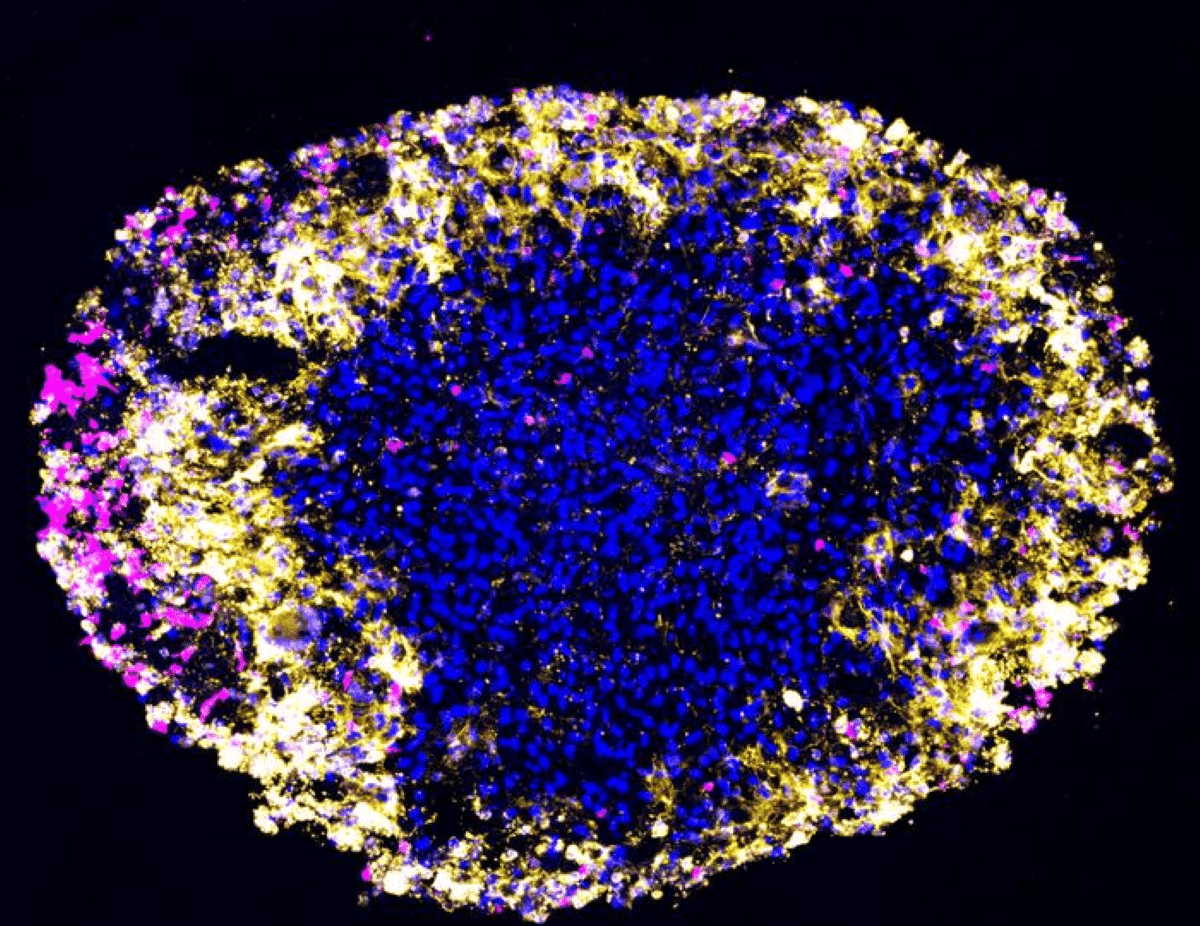
After reworking pores and skin cells into mind cells, the researchers discovered that the brand new neurons can develop in a skinny gel layer or self-assemble into small clusters — known as spheroids — mimicking the 3D setting of the mind.
The researchers in contrast neuronal spheroids generated from sufferers with sporadic, late-onset Alzheimer’s illness, inherited Alzheimer’s illness and wholesome people of comparable ages.
The Alzheimer’s illness sufferers’ spheroids rapidly developed amyloid beta deposits and tau tangles between neurons. Activation of genes related to irritation additionally emerged, after which the neurons started to die, mimicking what’s seen in mind scans of sufferers. Spheroids from older, wholesome donors within the research confirmed some amyloid deposition however a lot lower than these from sufferers.
The small amyloid deposits in older, wholesome spheroids are proof that the approach is capturing the consequences of age and counsel that amyloid beta and tau accumulation correlated with growing old. It additional demonstrates that the Alzheimer’s illness course of makes the buildup far worse.
The researchers, together with first writer Zhao Solar, PhD, a workers scientist in Yoo’s lab, discovered that treating spheroids from late-onset Alzheimer’s illness sufferers with medication that intervene with the formation of amyloid beta plaques early within the illness course of, earlier than neurons begin forming poisonous amyloid beta buildup, considerably lowered the amyloid beta deposits.
However treating at later time factors, after some buildup was already current, had no impact or solely modestly lowered subsequent amyloid beta deposits. Such knowledge emphasize the significance of figuring out and treating the illness early.
The research additional discovered a job for retrotransposable components — small items of DNA that leap to completely different places within the genome — within the growth of late-onset Alzheimer’s illness.
Inhibition of such “leaping genes” with the drug lamivudine (additionally known as 3TC) — an anti-retroviral drug that may dampen the exercise of retrotransposable components — had a optimistic impact: The spheroids from late-onset Alzheimer’s illness sufferers had lowered amyloid beta and tau tangles and confirmed much less neuronal loss of life in contrast with the identical spheroids handled with a placebo.
Lamivudine therapy had no useful impact on spheroids from sufferers with early-onset, inherited Alzheimer’s illness, offering proof that sporadic late-onset Alzheimer’s growth associated to growing old has distinct molecular options in contrast with inherited autosomal dominant Alzheimer’s illness.
“In these sufferers, our new mannequin system has recognized a job for retrotransposable components related to the illness course of,” Yoo mentioned.
“We have been happy to see that we might cut back the injury with a drug therapy that suppresses these components. We look ahead to utilizing this mannequin system as we work towards new personalised therapeutic interventions for late-onset Alzheimer’s illness.”
The researchers are planning future research with spheroids that embody a number of sorts of mind cells, together with neurons and glia.
About this Alzheimer’s illness analysis information
Creator: Jessica Church
Supply: WUSTL
Contact: Jessica Church – WUSTL
Picture: The picture is credited to Neuroscience Information
Authentic Analysis: Closed entry.
“Modeling late-onset Alzheimer’s illness neuropathology by way of direct neuronal reprogramming” by Andrew Yoo et al. Science
Summary
Modeling late-onset Alzheimer’s illness neuropathology by way of direct neuronal reprogramming
INTRODUCTION
Extracellular accumulation of amyloid-β (Aβ) deposits, insoluble tau formation, and neuronal loss are vital neuropathological hallmarks of Alzheimer’s illness (AD). Analysis on AD fashions has predominantly centered on genetic mutations linked to early-onset autosomal dominant AD (ADAD).
Nonetheless, the flexibility to mannequin the age-associated neuropathological options of sporadic late-onset AD (LOAD), accounting for over 95% of circumstances, stays a significant problem. This hole is as a result of complexity of LOAD stemming from numerous danger elements, together with growing old. Induced pluripotent stem cells have enabled the era of human neurons.
Nonetheless, these stem cell–primarily based neurons revert to a fetal-like mobile age, limiting their utility in reflecting age-associated traits. Alternatively, direct neuronal reprogramming of affected person somatic cells comparable to fibroblasts retains age-related traits.
We use brain-enriched microRNAs (miRNAs), miR-9/9*, and miR-124, as extremely environment friendly reprogramming effectors to generate LOAD neurons in three-dimensional (3D) setting as a strong platform for capturing vital age-associated AD phenotypes.
RATIONALE
Neurons generated by 3D-direct neuronal reprogramming of LOAD affected person fibroblasts would carry an identical genetic data and retain the mobile age of affected aged people. We thus hypothesize that miRNA-induced LOAD neurons would recapitulate age-associated degenerative processes characterised by late-onset neuropathological options of AD.
RESULTS
As proof of precept, cortical neurons have been generated by neuronal conversion of fibroblasts from people with ADAD utilizing miR-9/9*-124 together with NEUROD2 and MYT1L. MiRNA-induced neurons have been cultured in 3D environments consisting of (i) a skinny Matrigel layer embedded with AD neurons and (ii) excessive cell density, self-assembled spheroids comprised of instantly reprogrammed neurons.
AD phenotypes have been assessed compared to age-matched management neurons from cognitively regular people. We discovered that 3D ADAD neurons exhibited extracellular accumulation of Aβ, formation of seed-competent and insoluble tau, bulged dystrophic neurites, and neurodegeneration. Importantly, making use of this 3D neuronal reprogramming to fibroblasts from people with LOAD successfully manifested hallmark AD neuropathological options.
Notably, inhibiting APP processing in the course of the early part of neuronal reprogramming lowered the buildup of Aβ deposits, tauopathy, and neurodegeneration whereas therapy in the course of the late part when Aβ deposits had already begun to type was ineffective.
Moreover, LOAD neurons exhibited gene expression modifications associated to neuroinflammation in comparison with age-matched controls. Notably, each aged wholesome management (aged 66 to 90) and LOAD (aged 66 to 90) neurons manifested modifications in retrotransposon components (RTE) expression in comparison with younger wholesome management neurons (aged 36 to 61).
Disrupting age-associated RTE dysregulation in LOAD neurons utilizing lamivudine (3TC) led to the discount of Aβ, tau aggregation, neuronal loss of life, and DNA injury, correlated with expression modifications of genes related to irritation.
CONCLUSION
The findings exhibit the feasibility and sufficiency of miRNA-induced LOAD neurons for modeling late-onset neuropathology of AD in a 3D setting. These neurons present a platform to grasp how growing old influences vulnerability to late-onset neurodegeneration in LOAD sufferers.
Extending the present research, future analysis targets ought to be directed towards figuring out further growing old mechanisms contributing to AD pathogenesis, mechanisms associated to AD danger genes expressed in neurons, and interactions with different mind cell varieties that will affect pathological options of AD in patient-derived neurons.

















Discussion about this post